Wrecking neutrophil extracellular traps and antagonizing cancer-associated neurotransmitters by interpenetrating network hydrogels prevent postsurgical cancer relapse and metastases
- PMID: 38783926
- PMCID: PMC11112132
- DOI: 10.1016/j.bioactmat.2024.05.022
Wrecking neutrophil extracellular traps and antagonizing cancer-associated neurotransmitters by interpenetrating network hydrogels prevent postsurgical cancer relapse and metastases
Abstract
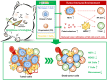
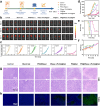
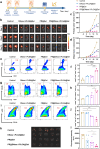
Tumor-promoting niche after incomplete surgery resection (SR) can lead to more aggressive local progression and distant metastasis with augmented angiogenesis-immunosuppressive tumor microenvironment (TME). Herein, elevated neutrophil extracellular traps (NETs) and cancer-associated neurotransmitters (CANTs, e.g., catecholamines) are firstly identified as two of the dominant inducements. Further, an injectable fibrin-alginate hydrogel with high tissue adhesion has been constructed to specifically co-deliver NETs inhibitor (DNase I)-encapsulated PLGA nanoparticles and an unselective β-adrenergic receptor blocker (propranolol). The two components (i.e., fibrin and alginate) can respond to two triggers (thrombin and Ca2+, respectively) in postoperative bleeding to gelate, shaping into an interpenetrating network (IPN) featuring high strength. The continuous release of DNase I and PR can wreck NETs and antagonize catecholamines to decrease microvessel density, blockade myeloid-derived suppressor cells, secrete various proinflammatory cytokines, potentiate natural killer cell function and hamper cytotoxic T cell exhaustion. The reprogrammed TME significantly suppress locally residual and distant tumors, induce strong immune memory effects and thus inhibit lung metastasis. Thus, targetedly degrading NETs and blocking CANTs enabled by this in-situ IPN-based hydrogel drug depot provides a simple and efficient approach against SR-induced cancer recurrence and metastasis.
Keywords: Cancer-associated neurotransmitters; Immunosuppressive tumor microenvironment; Interpenetrating network hydrogels; Neutrophil extracellular traps; Postsurgical cancer relapse and metastases.
© 2024 The Authors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Neutrophil Extracellular Traps Promote T Cell Exhaustion in the Tumor Microenvironment.Front Immunol. 2021 Nov 24;12:785222. doi: 10.3389/fimmu.2021.785222. eCollection 2021. Front Immunol. 2021. PMID: 34899751 Free PMC article.
-
Intraoperative Cavity Local Delivery System with NETs-Specific Drug Release for Post-Breast Cancer Surgery Recurrence Correction.Adv Healthc Mater. 2024 Dec;13(30):e2401537. doi: 10.1002/adhm.202401537. Epub 2024 Aug 29. Adv Healthc Mater. 2024. PMID: 39205549
-
Injectable adhesive hemostatic gel with tumor acidity neutralizer and neutrophil extracellular traps lyase for enhancing adoptive NK cell therapy prevents post-resection recurrence of hepatocellular carcinoma.Biomaterials. 2022 May;284:121506. doi: 10.1016/j.biomaterials.2022.121506. Epub 2022 Apr 1. Biomaterials. 2022. PMID: 35390709
-
Neutrophil Extracellular Traps (NETs) in Cancer Invasion, Evasion and Metastasis.Cancers (Basel). 2021 Sep 6;13(17):4495. doi: 10.3390/cancers13174495. Cancers (Basel). 2021. PMID: 34503307 Free PMC article. Review.
-
Neutrophil Extracellular Traps (NETs) in Cancer Metastasis.Cancers (Basel). 2021 Dec 6;13(23):6131. doi: 10.3390/cancers13236131. Cancers (Basel). 2021. PMID: 34885240 Free PMC article. Review.
Cited by
-
Disrupting calcium homeostasis and glycometabolism in engineered lipid-based pharmaceuticals propel cancer immunogenic death.Acta Pharm Sin B. 2025 Mar;15(3):1255-1267. doi: 10.1016/j.apsb.2024.12.018. Epub 2024 Dec 21. Acta Pharm Sin B. 2025. PMID: 40370551 Free PMC article.
-
Dynamically urethra-adapted and obligations-oriented trilayer hydrogels integrate scarless urethral repair.Nat Commun. 2025 Aug 8;16(1):7333. doi: 10.1038/s41467-025-62851-2. Nat Commun. 2025. PMID: 40781239 Free PMC article.
-
Myeloid cells meet CD8+ T cell exhaustion in cancer: What, why and how.Chin J Cancer Res. 2024 Dec 30;36(6):616-651. doi: 10.21147/j.issn.1000-9604.2024.06.04. Chin J Cancer Res. 2024. PMID: 39802897 Free PMC article.
-
[Research Progress of Neutrophil Extracellular Traps in Lung Cancer].Zhongguo Fei Ai Za Zhi. 2025 Mar 20;28(3):201-212. doi: 10.3779/j.issn.1009-3419.2025.106.06. Zhongguo Fei Ai Za Zhi. 2025. PMID: 40210480 Free PMC article. Review. Chinese.
-
pH-Responsive Modified HAMA Microspheres Regulate the Inflammatory Microenvironment of Intervertebral Discs.ACS Appl Mater Interfaces. 2024 Nov 20;16(46):63295-63305. doi: 10.1021/acsami.4c14475. Epub 2024 Nov 11. ACS Appl Mater Interfaces. 2024. PMID: 39529398 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

