Emicizumab is efficacious in people with hemophilia A with comorbidities aged ≥50 years: analysis of 4 phase III trials
- PMID: 38783987
- PMCID: PMC11112372
- DOI: 10.1016/j.rpth.2024.102405
Emicizumab is efficacious in people with hemophilia A with comorbidities aged ≥50 years: analysis of 4 phase III trials
Abstract
Background: The treatment of older people with hemophilia A (HA) can be complicated by comorbidities.
Objectives: This post hoc analysis evaluates the efficacy and safety of emicizumab in people with HA aged ≥50 years with cardiovascular (CV) risk factors or HIV and/or hepatitis C virus (HCV) infection.
Methods: The HAVEN 1 (NCT02622321), HAVEN 3 (NCT02847637), HAVEN 4 (NCT03020160), and STASEY (NCT03191799) studies enrolled adults/adolescents with severe HA. Participants were categorized as having a comorbidity if they had any CV risk factors (including history of CV disease, hypertension, diabetes, hyperlipidemia, prior stroke, or obesity), HIV, and/or HCV infection. Efficacy and safety outcomes were compared by age (<50 vs ≥50 years).
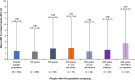
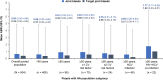
Results: Of 504 participants at data cutoff, 408 were aged <50 years and 96 were aged ≥50 years. In people with HA aged <50 years, 26.7% had ≥1 CV risk factor and 29.4% had HIV and/or HCV infection. In people with HA aged ≥50 years, 72.9% had ≥1 CV risk factor and 74.0% had HIV and/or HCV infection. The mean (95% CI) annualized bleed rate for treated bleeds was 1.29 (0.07-6.06) for people with HA aged <50 years and 1.82 (0.19-6.93) for people with HA aged ≥50 years. No significant differences in annualized bleed rates were observed for those with comorbidities compared with those without. Safety outcomes were similar regardless of age.
Conclusion: This pooled analysis suggests that emicizumab efficacy and safety in people with HA aged ≥50 years with CV and HIV/HCV comorbidities were consistent with those in people with HA aged <50 years enrolled in the HAVEN 1, 3, and 4 and STASEY studies.
Keywords: comorbidities; comorbidity; emicizumab; hemophilia A; prophylaxis.
© 2024 The Authors.
Figures
Similar articles
-
Long-term outcomes with emicizumab in hemophilia A without inhibitors: results from the HAVEN 3 and 4 studies.Res Pract Thromb Haemost. 2024 Mar 1;8(2):102364. doi: 10.1016/j.rpth.2024.102364. eCollection 2024 Feb. Res Pract Thromb Haemost. 2024. PMID: 38559572 Free PMC article.
-
Emicizumab use in females with moderate or mild hemophilia A without factor VIII inhibitors who warrant prophylaxis.Res Pract Thromb Haemost. 2023 Oct 21;7(8):102239. doi: 10.1016/j.rpth.2023.102239. eCollection 2023 Nov. Res Pract Thromb Haemost. 2023. PMID: 38193069 Free PMC article.
-
Efficacy, safety, and pharmacokinetics of emicizumab prophylaxis given every 4 weeks in people with haemophilia A (HAVEN 4): a multicentre, open-label, non-randomised phase 3 study.Lancet Haematol. 2019 Jun;6(6):e295-e305. doi: 10.1016/S2352-3026(19)30054-7. Epub 2019 Apr 16. Lancet Haematol. 2019. PMID: 31003963 Clinical Trial.
-
Bispecific Antibody Emicizumab for Haemophilia A: A Breakthrough for Patients with Inhibitors.BioDrugs. 2018 Dec;32(6):561-570. doi: 10.1007/s40259-018-0315-0. BioDrugs. 2018. PMID: 30430367 Review.
-
Emicizumab is well tolerated and effective in people with congenital hemophilia A regardless of age, severity of disease, or inhibitor status: a scoping review.Res Pract Thromb Haemost. 2024 Apr 18;8(4):102415. doi: 10.1016/j.rpth.2024.102415. eCollection 2024 May. Res Pract Thromb Haemost. 2024. PMID: 38812987 Free PMC article.
Cited by
-
Haemophilia Prophylaxis in the Age of Innovation: Exploring Opportunities for Personalized Treatment.Haemophilia. 2025 Jul;31(4):607-616. doi: 10.1111/hae.70015. Epub 2025 Apr 17. Haemophilia. 2025. PMID: 40242991 Free PMC article. Review.
References
-
- Srivastava A., Elena S., Dougall A., Kitchen S., Sutherland M., Pipe S.W., et al. WFH Guidelines for the management of hemophilia, 3rd edition. Haemophilia. 2020;26(suppl 6):1–158. - PubMed
-
- Shapiro S., Makris M. Haemophilia and ageing. Br J Haematol. 2019;184:712–720. - PubMed
-
- Blair H.A. Emicizumab: a review in haemophilia A. Drugs. 2019;79:1697–1707. - PubMed
LinkOut - more resources
Full Text Sources

