VDAC1-interacting molecules promote cell death in cancer organoids through mitochondrial-dependent metabolic interference
- PMID: 38784007
- PMCID: PMC11112339
- DOI: 10.1016/j.isci.2024.109853
VDAC1-interacting molecules promote cell death in cancer organoids through mitochondrial-dependent metabolic interference
Erratum in
-
Erratum: VDAC1-interacting molecules promote cell death in cancer organoids through mitochondrial-dependent metabolic interference.iScience. 2025 Mar 27;28(4):112242. doi: 10.1016/j.isci.2025.112242. eCollection 2025 Apr 18. iScience. 2025. PMID: 40224015 Free PMC article.
Abstract
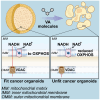
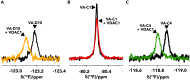
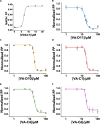
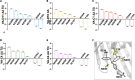
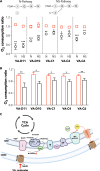
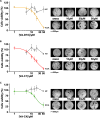
The voltage-dependent anion-selective channel isoform 1 (VDAC1) is a pivotal component in cellular metabolism and apoptosis with a prominent role in many cancer types, offering a unique therapeutic intervention point. Through an in-silico-to-in-vitro approach we identified a set of VA molecules (VDAC Antagonists) that selectively bind to VDAC1 and display specificity toward cancer cells. Biochemical characterization showed that VA molecules can directly interact with VDAC1 with micromolar affinity by competing with the endogenous ligand NADH for a partially shared binding site. NADH displacement results in mitochondrial distress and reduced cell proliferation, especially when compared to non-cancerous cells. Experiments performed on organoids derived from intrahepatic cholangiocarcinoma patients demonstrated a dose-dependent reduction in cell viability upon treatment with VA molecules with lower impact on healthy cells than conventional treatments like gemcitabine. VA molecules are chemical entities representing promising candidates for further optimization and development as cancer therapy strategies through precise metabolic interventions.
Keywords: Functional aspects of cell biology; Molecular medicine; Small molecule.
© 2024 The Authors.
Conflict of interest statement
M.H.A. is an employee of Atomwise, Inc., San Francisco, CA, USA.
Figures



References
LinkOut - more resources
Full Text Sources

