Enhancing genetic variability in Trigonella species through sodium azide induction: morpho-physiological and chromosomal amelioration
- PMID: 38784032
- PMCID: PMC11111941
- DOI: 10.3389/fgene.2024.1378368
Enhancing genetic variability in Trigonella species through sodium azide induction: morpho-physiological and chromosomal amelioration
Abstract
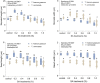
Plant breeding, aimed at enhancing desired traits, depends on genetic diversity. Mutation breeding is a powerful method of rapidly expanding genetic diversity, facilitating crop improvement, and ensuring food security. In a recent study, researchers evaluated the genetic variability of Trigonella species using different doses of sodium azide (SA) (0.2%, 0.4%, 0.6%, 0.8%, and 1.0%) through morphological, physiological, and cytogenetic studies. Morphological variations were observed in cotyledonary leaves, vegetative leaves, and overall plant growth and habit. Several quantitative parameters, such as plant height, fertile branches per plant, pods per plant (or clusters), seeds per pod, and seed yield, increased when treated with 0.2% and 0.4% SA compared to the control. Furthermore, the total chlorophyll content and carotenoids increased in the sample treated with 0.2% SA over the control but decreased with higher concentrations. Scanning electron microscopy revealed that stomatal aperture and seed dimensions increased at lower concentrations of sodium azide treatment. The study found a positive correlation between the different parameters studied in the Trigonella species, as indicated by high r-values. Based on their findings, it was concluded that the genotype of fenugreek can be improved by using 0.2% and 0.4% concentrations of sodium azide. However, the evaluation of observed variants in successive generations is a critical and necessary process to validate their potential as keystones for crop genetic improvements.
Keywords: chromosomal alterations; crop genetic improvement; fenugreek genotype; genetic diversity; plant breeding.
Copyright © 2024 Naaz, Choudhary, Hasan, Sharma, Alharbi and Abd El Moneim.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures








References
-
- Agrawal L., Kumar M. (2021). Improvement in ornamental, medicinal, and aromatic plants through induced mutation. J. Appl. Biol. Biotechnol. 9 (4), 1–6. 10.7324/JABB.2021.9422 - DOI
-
- Ahire D., Auti S. (2015). Effect of chemical and physical mutagen in M1 generation and chlorophyll mutations in soybean (Glycine max L. Merrill). Int. J. Bioassayas 4, 4235–4240.
-
- Al-Qurainy F. (2009). Toxicity of heavy metals and their molecular detection on Phaseolus vulgaris (L.). Aust. J. basic Appl. Sci. 3 (3), 3025–3035.
-
- Aman R. D. (1986). A model of seed dormancy. Bot. Rev. 34, 1–31. 10.1007/BF02858619 - DOI
-
- Amin R. W., RainaKhursheed A. S., Khan S. (2019). Induced morphological and chromosomal diversity in the mutagenized population of black cumin (Nigella sativa L.) using single and combination treatments of gamma rays and ethyl methane sulfonate. Jordan J. Biol. Sci. 12, 1.
LinkOut - more resources
Full Text Sources

