Dyslipidaemia management in pregnant patients: a 2024 update
- PMID: 38784103
- PMCID: PMC11114474
- DOI: 10.1093/ehjopen/oeae032
Dyslipidaemia management in pregnant patients: a 2024 update
Abstract
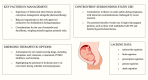
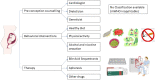
Over several decades, the approach to treating dyslipidaemias during pregnancy remains essentially unchanged. The lack of advancement in this field is mostly related to the fact that we lack clinical trials of pregnant patients both with available as well as new therapies. While there are numerous novel therapies developed for non-pregnant patients, there are still many limitations in dyslipidaemia treatment during pregnancy. Besides pharmacotherapy and careful clinical assessment, the initiation of behavioural modifications as well as pre-conception management is very important. Among the various lipid-lowering medications, bile acid sequestrants are the only ones officially approved for treating dyslipidaemia in pregnancy. Ezetimibe and fenofibrate can be considered if their benefits outweigh potential risks. Statins are still considered contraindicated, primarily due to animal studies and human case reports. However, recent systematic reviews and meta-analyses as well as data on familial hypercholesterolaemia (FH) in pregnant patients have indicated that their use may not be harmful and could even be beneficial in certain selected cases. This is especially relevant for pregnant patients at very high cardiovascular risk, such as those who have already experienced an acute cardiovascular event or have homozygous or severe forms of heterozygous FH. In these cases, the decision to continue therapy during pregnancy should weigh the potential risks of discontinuation. Bempedoic acid, olezarsen, evinacumab, evolocumab and alirocumab, and inclisiran are options to consider just before and after pregnancy is completed. In conclusion, decisions regarding lipid-lowering therapy for pregnant patients should be personalized. Despite the challenges in designing and conducting studies in pregnant women, there is a strong need to establish the safety and efficacy of dyslipidaemia treatment during pregnancy.
Keywords: Bile acid sequestrants; Dyslipidaemia; Familial hypercholesterolaemia; Pregnancy; Statins.
© The Author(s) 2024. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: none declared.
Figures
References
-
- Hadden DR, McLauglin C. Normal and abnormal maternal metabolism during pregnancy. Semin Fetal Neonatal Med 2009;14:401. - PubMed
-
- Vrijkotte TG, Krukziener N, Hutten BA, Vollebregt KC, van Eijsden M, Twickler MB. Maternal lipid profile during early pregnancy and pregnancy complications and outcomes: the ABCD study. J Clin Endocrinol Metab 2012;97:3917–3925. - PubMed
-
- Pipe NG, Smith T, Halliday D, Edmonds CJ, Williams C, Coltart TM. Changes in fat, fat-free mass and body water in human normal pregnancy. Br J Obstet Gynaecol 1979;86:929–940. - PubMed
-
- Clapp JF, Seaward BL 3rd, Sleamaker RH, Hiser J. Maternal physiologic adaptations to early human pregnancy. Am J Obstet Gynecol 1988;159:1456–1460. - PubMed
-
- Kopp-Hoolihan LE, van Loan MD, Wong WW, King JC. Fat mass deposition during pregnancy using a four-component model. J Appl Physiol 1999;87:196–202. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous