Disseminated Cryptococcosis Post Eculizumab Therapy: A Case Report and Literature Review
- PMID: 38784297
- PMCID: PMC11115998
- DOI: 10.7759/cureus.58852
Disseminated Cryptococcosis Post Eculizumab Therapy: A Case Report and Literature Review
Abstract
Eculizumab is a biologic medication used for the treatment of complement-related disorders including anti-acetylcholine receptor antibody-positive generalized myasthenia gravis. It targets C5 complement, preventing its cleavage into active terminal components. Thus, vaccination against encapsulated organisms is advised before starting this treatment. C5 also has a critical role against Cryptococcus neoformans infection. Here, we present a case of a 34-year-old man with a history of myasthenia gravis who was treated with prednisone and azathioprine in addition to eculizumab that was added to his regimen about a year ago, and who came to the hospital with headache, and was found to have Cryptococcus meningitis with disseminated cryptococcosis. The patient was negative for human immunodeficiency virus. He was treated with antifungal medications, and his condition improved. Although rarely reported, it is important to have a low threshold for diagnosis of cryptococcosis in patients on eculizumab given its complement inhibition mechanism of action.
Keywords: complement c5; cryptococcus neoformans; eculizumab; fungal meningitis; myasthenia gravis (mg).
Copyright © 2024, Youssef et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
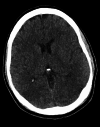
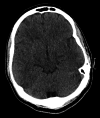
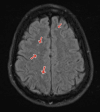
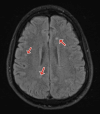
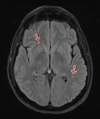
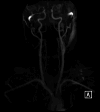
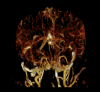
Similar articles
-
Disseminated cryptococcosis associated with administration of eculizumab.Am J Health Syst Pharm. 2018 Jul 15;75(14):1018-1022. doi: 10.2146/ajhp170708. Epub 2018 Jun 12. Am J Health Syst Pharm. 2018. PMID: 29895518
-
Safety and efficacy of eculizumab in anti-acetylcholine receptor antibody-positive refractory generalised myasthenia gravis (REGAIN): a phase 3, randomised, double-blind, placebo-controlled, multicentre study.Lancet Neurol. 2017 Dec;16(12):976-986. doi: 10.1016/S1474-4422(17)30369-1. Epub 2017 Oct 20. Lancet Neurol. 2017. PMID: 29066163 Clinical Trial.
-
Novel uses of complement inhibitors in myasthenia gravis-Two case reports.Muscle Nerve. 2024 Mar;69(3):368-372. doi: 10.1002/mus.28037. Epub 2024 Jan 11. Muscle Nerve. 2024. PMID: 38205840
-
Pleural effusion as the initial clinical presentation in disseminated cryptococcosis and fungaemia: an unusual manifestation and a literature review.BMC Infect Dis. 2015 Sep 22;15:385. doi: 10.1186/s12879-015-1132-4. BMC Infect Dis. 2015. PMID: 26395579 Free PMC article. Review.
-
Eculizumab treatment for myasthenia gravis subgroups: 2021 update.J Neuroimmunol. 2022 Jan 15;362:577767. doi: 10.1016/j.jneuroim.2021.577767. Epub 2021 Nov 18. J Neuroimmunol. 2022. PMID: 34823117 Review.
Cited by
-
New and Emerging Biological Therapies for Myasthenia Gravis: A Focussed Review for Clinical Decision-Making.BioDrugs. 2025 Mar;39(2):185-213. doi: 10.1007/s40259-024-00701-1. Epub 2025 Jan 27. BioDrugs. 2025. PMID: 39869260 Free PMC article. Review.
References
-
- Eculizumab in Aquaporin-4-positive neuromyelitis optica spectrum disorder. Pittock SJ, Berthele A, Fujihara K, et al. N Engl J Med. 2019;381:614–625. - PubMed
-
- Management of infection in PNH patients treated with eculizumab or other complement inhibitors: unmet clinical needs. Girmenia C, Barcellini W, Bianchi P, et al. Blood Rev. 2023;58:101013. - PubMed
-
- Role for C5 and neutrophils in the pulmonary intravascular clearance of circulating Cryptococcus neoformans. Lovchik JA, Lipscomb MF. Am J Respir Cell Mol Biol. 1993;9:617–627. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
