C(sp3)-H cyclizations of 2-(2-vinyl)phenoxy- tert-anilines
- PMID: 38784409
- PMCID: PMC11112676
- DOI: 10.1039/d3ra08974f
C(sp3)-H cyclizations of 2-(2-vinyl)phenoxy- tert-anilines
Abstract
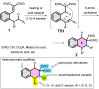
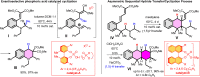
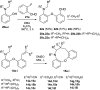
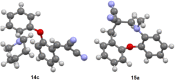
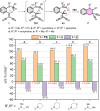
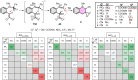
1,5-hydride transfer-triggered cyclization reactions offering a robust method for C(sp3)-C(sp3) coupling and the synthesis of e.g. tetrahydroquinolines have been thoroughly investigated in the literature. Catalysts allowing milder reaction conditions or the development of enantioselective processes were important recent contributions to the field, as well as the studies on subtrates with oxygen or sulfur heteroatoms (besides the originally described nitrogen heterocycles). In a series of studies, we focused on expanded, higher order H-transfers/cyclizations by positioning the interacting substituents on distanced rings. Cyclizations of appropriately functionalized biaryl and fused bicyclic systems led to 7-9 membered rings. In the frame of this research, we set out to study the feasibility of the cyclization and the factors affecting it by in silico methods. The conclusions drawn from computational studies were complemented by cyclization screens on 2-(2-vinyl)phenoxy-tert-anilines and their CH2-expanded analogues, the results of which are presented here. Besides isolating the expected oxazonine products in several cases, we also observed a unique dimer formation, leading to an interesting 5-6-5 ring system.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures





References
-
- Gutekunst W. R. Baran P. S. C–H functionalization logic in total synthesis. Chem. Soc. Rev. 2011;40:1976–1991. - PubMed
-
- Yamaguchi J. Yamaguchi A. D. Itami K. C–H Bond Functionalization: Emerging Synthetic Tools for Natural Products and Pharmaceuticals. Angew. Chem., Int. Ed. 2012;51:8960–9009. - PubMed
-
- Haibach M. C. Seidel D. C–H Bond Functionalization through Intramolecular Hydride Transfer. Angew. Chem., Int. Ed. 2014;53:5010–5036. - PubMed
LinkOut - more resources
Full Text Sources

