Early Real-World Patient and Staff Experience with an Intracanalicular Dexamethasone Insert
- PMID: 38784434
- PMCID: PMC11114144
- DOI: 10.2147/OPTH.S448973
Early Real-World Patient and Staff Experience with an Intracanalicular Dexamethasone Insert
Erratum in
-
Erratum: Early Real-World Patient and Staff Experience With an Intracanalicular Dexamethasone Insert [Corrigendum].Clin Ophthalmol. 2025 Feb 4;19:385-386. doi: 10.2147/OPTH.S519248. eCollection 2025. Clin Ophthalmol. 2025. PMID: 39931679 Free PMC article.
Abstract
Purpose: To evaluate both the early experience of real-world patients treated with dexamethasone ophthalmic insert (0.4 mg; DEXTENZA®), hereafter referred to as DEX, after cataract surgery as well as staff/practice integration of DEX relative to eyedrops.
Patients and methods: This was a cross-sectional survey study of 23 cataract practices in the United States. Respondents were patients and practice staff who had experience with DEX following cataract surgery. Both patients and practice staff completed an online survey. Descriptive statistics summarized the survey responses to portray the experience of the respondents.
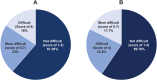
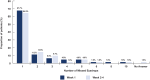
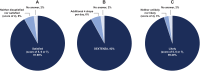
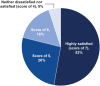
Results: Surveys were completed by 62 patients and 19 practice staff. Almost all patients (93%) were satisfied or extremely satisfied with DEX. Patients highly preferred DEX (93%) to topical steroid drops (7%) based on past experiences with topical steroid drops. Most practice staff (95%) were satisfied or highly satisfied with DEX, reporting a 45% reduction in time spent educating patients on postoperative drop use and a 46% decrease in time spent addressing calls from pharmacies regarding postoperative medications.
Conclusion: Incorporating the DEX insert into clinical practice in cataract surgery practices can improve patient adherence, while potentially providing significant savings to practices in terms of time spent educating patients and responding to patient and pharmacy call-backs.
Keywords: hands-free therapy; intracanalicular dexamethasone insert; ocular inflammation; ocular pain; phacoemulsification; sustained-release drug delivery.
© 2024 Nijm et al.
Conflict of interest statement
Lisa Nijm, Cynthia Matossian, John D Stephens, Parag A Majmudar, Subba Rao Gollamudi, Ravi H Patel, and Maria E Rosselson received support from Ocular Therapeutix, Inc. for their participation in the study. Aditi Bauskar, Alyssa Montieth, Srilatha Vantipalli, Jamie Lynne Metzinger, and Rabia Ozden are employees of Ocular Therapeutix, Inc. Michelle K Rhee reports grants from Ocular Therapeutix; on advisory board for NovaBay and Nevakar, medical director for The Eye-Bank for Sight Restoration, outside the submitted work; Research support from Ocular Therapeutix, Inc. Andrea Gibson and Michael H Goldstein are consultants for and former employees of Ocular Therapeutix, Inc. The authors report no other conflicts of interest in this work.
Figures


References
LinkOut - more resources
Full Text Sources
Miscellaneous

