Cupric-polymeric nanoreactors integrate into copper metabolism to promote chronic diabetic wounds healing
- PMID: 38784443
- PMCID: PMC11111831
- DOI: 10.1016/j.mtbio.2024.101087
Cupric-polymeric nanoreactors integrate into copper metabolism to promote chronic diabetic wounds healing
Abstract
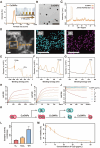
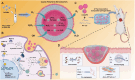
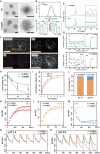
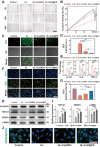
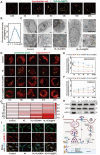
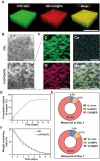
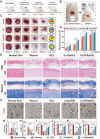
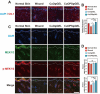
Given multifunction of copper (Cu) contributing to all stages of the physiology of wound healing, Cu-based compounds have great therapeutic potentials to accelerate the wound healing, but they must be limited to a very low concentration range to avoid detrimental accumulation. Additionally, the cellular mechanism of Cu-based compounds participating the healing process remains elusive. In this study, copper oxide nanoparticles (CuONPs) were synthesized to mimic the multiple natural enzymes and trapped into PEG-b-PCL polymersomes (PS) to construct cupric-polymeric nanoreactors (CuO@PS) via a direct hydration method, thus allowing to compartmentalize Cu-based catalytic reactions in an isolated space to improve the efficiency, selectivity, recyclability as well as biocompatibility. While nanoreactors trafficked to lysosomes following endocytosis, the released Cu-based compounds in lysosomal lumen drove a cytosolic Cu+ influx to mobilize Cu metabolism mostly via Atox1-ATP7a/b-Lox axis, thereby activating the phosphorylation of mitogen-activated protein kinase 1 and 2 (MEK1/2) to initiate downstream signaling events associated with cell proliferation, migration and angiogenesis. Moreover, to facilitate to lay on wounds, cupric-polymeric nanoreactors were finely dispersed into a thermosensitive Pluronic F127 hydrogel to form a composite hydrogel sheet that promoted the healing of chronic wounds in diabetic rat models. Hence, cupric-polymeric nanoreactors represented an attractive translational strategy to harness cellular Cu metabolism for chronic wounds healing.
Keywords: Atox1-ATP7a/b-Lox axis; Chronic wound healing; Copper metabolism; Cupric-polymeric nanoreactors; MEK1/2 phosphorylation.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
Preparation of Ultra-Small Copper Nanoparticles-Loaded Self-Healing Hydrogels with Antibacterial, Inflammation-Suppressing and Angiogenesis-Enhancing Properties for Promoting Diabetic Wound Healing.Int J Nanomedicine. 2023 Jun 19;18:3339-3358. doi: 10.2147/IJN.S399933. eCollection 2023. Int J Nanomedicine. 2023. PMID: 37361387 Free PMC article.
-
Endothelial Antioxidant-1: a Key Mediator of Copper-dependent Wound Healing in vivo.Sci Rep. 2016 Sep 26;6:33783. doi: 10.1038/srep33783. Sci Rep. 2016. PMID: 27666810 Free PMC article.
-
Antibacterial adhesive self-healing hydrogels to promote diabetic wound healing.Acta Biomater. 2022 Jul 1;146:119-130. doi: 10.1016/j.actbio.2022.04.041. Epub 2022 Apr 26. Acta Biomater. 2022. PMID: 35483628
-
Natural Polymeric Hydrogels Encapsulating Small Molecules for Diabetic Wound Healing.Gels. 2023 Oct 30;9(11):867. doi: 10.3390/gels9110867. Gels. 2023. PMID: 37998956 Free PMC article. Review.
-
[The modern approach to wound treatment].Med Pregl. 2000 Jul-Aug;53(7-8):363-8. Med Pregl. 2000. PMID: 11214479 Review. Croatian.
Cited by
-
A one-two punch of inflammation and oxidative stress promotes revascularization for diabetic foot ulcers.Mater Today Bio. 2025 Feb 4;31:101548. doi: 10.1016/j.mtbio.2025.101548. eCollection 2025 Apr. Mater Today Bio. 2025. PMID: 39995578 Free PMC article.
-
The Journey of Copper-Impregnated Dressings in Wound Healing: From a Medical Hypothesis to Clinical Practice.Biomedicines. 2025 Feb 24;13(3):562. doi: 10.3390/biomedicines13030562. Biomedicines. 2025. PMID: 40149539 Free PMC article. Review.
-
FGF mimetic peptide-modified electrospun nanocomposite fibrous membranes for accelerating infectious diabetic wound healing by synergistic antibacterial and pro-angiogenesis effects.Mater Today Bio. 2025 May 17;32:101877. doi: 10.1016/j.mtbio.2025.101877. eCollection 2025 Jun. Mater Today Bio. 2025. PMID: 40496728 Free PMC article.
References
-
- Bowers S., Franco E. Chronic wounds: Evaluation and management. Am. Fam. Physician. 2020;101:159–166. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

