Chiral hydroxymethyl-1 H,3 H-pyrrolo[1,2- c]thiazoles: the search for selective p53-activating agents for colorectal cancer therapy
- PMID: 38784460
- PMCID: PMC11110798
- DOI: 10.1039/d4md00076e
Chiral hydroxymethyl-1 H,3 H-pyrrolo[1,2- c]thiazoles: the search for selective p53-activating agents for colorectal cancer therapy
Erratum in
-
Correction: Chiral hydroxymethyl-1H,3H-pyrrolo[1,2-c]thiazoles: the search for selective p53-activating agents for colorectal cancer therapy.RSC Med Chem. 2025 Feb 3;16(2):970. doi: 10.1039/d5md90004b. eCollection 2025 Feb 19. RSC Med Chem. 2025. PMID: 39906312 Free PMC article.
Abstract
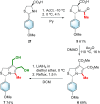
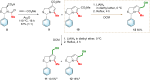
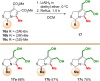
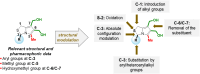
MANIO is an efficient p53-activating anticancer agent with remarkable selectivity to the p53 pathway and promising antitumor activity against colorectal cancer (CRC). Herein, a library of novel MANIO derivatives, including hydroxymethyl- and bis(hydroxymethyl)-1H,3H-pyrrolo[1,2-c]thiazoles, was synthesized by rational structural modulation. The antiproliferative activity of twenty derivatives was evaluated in a panel of human CRC cells with different p53 status. From this library, five compounds with R- and S-configuration and with aromatic or heteroaromatic groups at position 3, including the enantiomer of MANIO, were identified as selective towards p53-expressing cancer cells. On the other hand, two compounds with S-configuration, 6-hydroxymethyl- and 7-hydroxymethyl-5-methyl-3-phenyl-1H,3H-pyrrolo[1,2-c]thiazoles, showed high cytotoxicity against WTp53-expressing HCT116 colon cells but, unlike MANIO, exhibited p53-independent inhibitory activity in CRC. The results described provide relevant structural and pharmacophoric data for the design of new p53-activating agents for precision therapy of CRC or other p53-related cancers harboring both wild-type or mutated p53 forms.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
One patent application protecting the compounds disclosed in this manuscript has been filed by the following authors M. I. L. S., L. S., and T. M. V. D. P. M.
Figures





References
-
- Prager G. W. Braga S. Bystricky B. Qvortrup C. Criscitiello C. Esin E. Sonke G. S. Martínez G. A. Frenel J. S. Karamouzis M. Strijbos M. Yazici O. Bossi P. Banerjee S. Troiani T. Eniu A. Ciardiello F. Tabernero J. Zielinski C. C. Casali P. G. Cardoso F. Douillard J. Y. Jezdic S. McGregor K. Bricalli G. Vyas M. Ilbawi A. ESMO Open. 2018;3:e000285. doi: 10.1136/esmoopen-2017-000285. - DOI - PMC - PubMed
-
- WHO report on cancer: setting priorities, investing wisely and providing care for all, World Health Organization, Geneva, 2020
-
- Ramos H. Soares M. I. L. Silva J. Raimundo L. Calheiros J. Gomes C. Reis F. Monteiro F. A. Nunes C. Reis S. Bosco B. Piazza S. Domingues L. Chlapek P. Vlcek P. Fabian P. Rajado A. T. Carvalho A. T. P. Veselska R. Inga A. Pinho e Melo T. M. V. D. Saraiva L. Cell Rep. 2021;35:108982. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

