Boron-containing helicenes as new generation of chiral materials: opportunities and challenges of leaving the flatland
- PMID: 38784742
- PMCID: PMC11110153
- DOI: 10.1039/d4sc01083c
Boron-containing helicenes as new generation of chiral materials: opportunities and challenges of leaving the flatland
Abstract
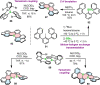
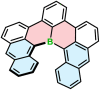
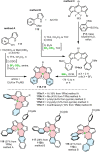
Increased interest in chiral functional dyes has stimulated activity in the field of boron-containing helicenes over the past few years. Despite the fact that the introduction of boron endows π-conjugated scaffolds with attractive electronic and optical properties, boron helicenes have long remained underdeveloped compared to other helicenes containing main group elements. The main reason was the lack of reliable synthetic protocols to access these scaffolds. The construction of boron helicenes proceeds against steric strain, and thus the methods developed for planar systems have sometimes proven ineffective in their synthesis. Recent advances in the general boron chemistry and the synthesis of strained derivatives have opened the way to a wide variety of boron-containing helicenes. Although the number of helically chiral derivatives is still limited, these compounds are currently at the forefront of emissive materials for circularly-polarized organic light-emitting diodes (CP-OLEDs). Yet the design of good emitters is not a trivial task. In this perspective, we discuss a number of requirements that must be met to provide an excellent emissive material. These include chemical and configurational stability, emission quantum yields, luminescence dissymmetry factors, and color purity. Understanding of these parameters and some structure-property relationships should aid in the rational design of superior boron helicenes. We also present the main achievements in their synthesis and point out niches in this area, e.g. stereoselective synthesis, necessary to accelerate the development of this fascinating class of compounds and to realize their potential in OLED devices and in other fields.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures














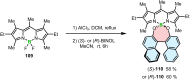


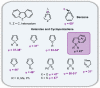

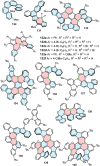





References
-
- Martin R. H. Angew. Chem., Int. Ed. 1974;13:649–660. doi: 10.1002/anie.197406491. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

