Enantioselective synthesis of α-aryl α-hydrazino phosphonates
- PMID: 38784752
- PMCID: PMC11110148
- DOI: 10.1039/d4sc00822g
Enantioselective synthesis of α-aryl α-hydrazino phosphonates
Abstract
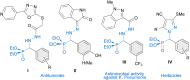
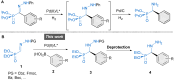
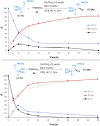
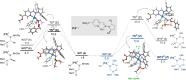
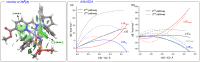
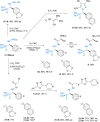
Catalysts generated in situ by the combination of pyridine-hydrazone N,N-ligands and Pd(TFA)2 have been applied to the addition of arylboronic acids to formylphosphonate-derived hydrazones, yielding α-aryl α-hydrazino phosphonates in excellent enantioselectivities (96 → 99% ee). Subsequent removal of the benzyloxycarbonyl (Cbz) N-protecting group afforded key building blocks en route to appealing artificial peptides, herbicides and antitumoral derivatives. Experimental and computational data support a stereochemical model based on aryl-palladium intermediates in which the phosphono hydrazone coordinates in its Z-configuration, maximizing the interactions between the substrate and the pyridine-hydrazone ligand.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

References
-
- Shie J.-J. Fang J.-M. Lai P.-T. Wen W.-H. Wang S.-Y. Cheng Y.-S. E. Tsai K.-C. Yang A.-S. Wong C.-H. J. Am. Chem. Soc. 2011;133:17959. doi: 10.1021/ja207892q. - DOI - PubMed
- Shie J.-J. Fang J.-M. Wang S.-Y. Tsai K.-C. Cheng Y.-S. E. Yang A.-S. Hsiao S.-C. Su C.-Y. Wong C.-H. J. Am. Chem. Soc. 2007;129:11892. doi: 10.1021/ja073992i. - DOI - PubMed
- Wang P.-C. Fang J.-M. Tsai K.-C. Wang S.-Y. Huang W.-I. Tseng Y.-C. Cheng Y.-S. E. Cheng T.-J. R. Wong C.-H. J. Med. Chem. 2016;59:5297. doi: 10.1021/acs.jmedchem.6b00029. - DOI - PubMed
-
- Koszelewski D. Kowalczyk P. Śmigielski P. Samsonowicz-Górski J. Kramkowski K. Wypych A. Szymczak M. Ostaszewski R. Materials. 2022;15:3846. doi: 10.3390/ma15113846. - DOI - PMC - PubMed
- Nowak M. G. Skwarecki A. S. Milewska M. J. ChemMedChem. 2021;16:3513. doi: 10.1002/cmdc.202100503. - DOI - PMC - PubMed
-
- Rao K. U. M. Swapna S. Manidhar D. M. Reddy K. M. K. Reddy C. S. Phosphorus, Sulfur Silicon Relat. Elem. 2015;190:232. doi: 10.1080/10426507.2014.914937. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

