Influence of the substitution position on spin communication in photoexcited perylene-nitroxide dyads
- PMID: 38784753
- PMCID: PMC11110163
- DOI: 10.1039/d4sc00328d
Influence of the substitution position on spin communication in photoexcited perylene-nitroxide dyads
Abstract
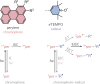
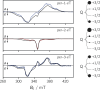
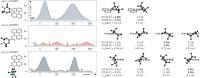
By virtue of the modularity of their structures, their tunable optical and magnetic properties, and versatile applications, photogenerated triplet-radical systems provide an ideal platform for the study of the factors controlling spin communication in molecular frameworks. Typically, these compounds consist of an organic chromophore covalently attached to a stable radical. After formation of the chromophore triplet state by photoexcitation, two spin centres are present in the molecule that will interact. The nature of their interaction is governed by the magnitude of the exchange interaction between them and can be studied by making use of transient electron paramagnetic resonance (EPR) techniques. Here, we investigate three perylene-nitroxide dyads that only differ with respect to the position where the nitroxide radical is attached to the perylene core. The comparison of the results from transient UV-vis and EPR experiments reveals major differences in the excited state properties of the three dyads, notably their triplet state formation yield, excited state deactivation kinetics, and spin coherence times. Spectral simulations and quantum chemical calculations are used to rationalise these findings and demonstrate the importance of considering the structural flexibility and the contribution of rotational conformers for an accurate interpretation of the data.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



References
LinkOut - more resources
Full Text Sources

