An adsorbate biased dynamic 3D porous framework for inverse CO2 sieving over C2H2
- PMID: 38784756
- PMCID: PMC11110155
- DOI: 10.1039/d3sc06611h
An adsorbate biased dynamic 3D porous framework for inverse CO2 sieving over C2H2
Abstract
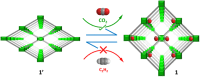
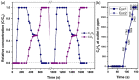
Separating carbon dioxide (CO2) from acetylene (C2H2) is one of the most critical and complex industrial separations due to similarities in physicochemical properties and molecular dimensions. Herein, we report a novel Ni-based three-dimensional framework {[Ni4(μ3-OH)2(μ2-OH2)2(1,4-ndc)3](3H2O)}n (1,4-ndc = 1,4-naphthalenedicarboxylate) with a one-dimensional pore channel (3.05 × 3.57 Å2), that perfectly matches with the molecular size of CO2 and C2H2. The dehydrated framework shows structural transformation, decorated with an unsaturated Ni(ii) centre and pendant oxygen atoms. The dynamic nature of the framework is evident by displaying a multistep gate opening type CO2 adsorption at 195, 273, and 298 K, but not for C2H2. The real time breakthrough gas separation experiments reveal a rarely attempted inverse CO2 selectivity over C2H2, attributed to open metal sites with a perfect pore aperture. This is supported by crystallographic analysis, in situ spectroscopic inspection, and selectivity approximations. In situ DRIFTS measurements and DFT-based theoretical calculations confirm CO2 binding sites are coordinatively unsaturated Ni(ii) and carboxylate oxygen atoms, and highlight the influence of multiple adsorption sites.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures





Similar articles
-
A Scalable Pore-space-partitioned Metal-organic Framework Powered by Polycatenation Strategy for Efficient Acetylene Purification.Angew Chem Int Ed Engl. 2025 Feb 3;64(6):e202421992. doi: 10.1002/anie.202421992. Epub 2024 Dec 23. Angew Chem Int Ed Engl. 2025. PMID: 39668752
-
Reverse Separation of Carbon Dioxide and Acetylene in Two Isostructural Copper Pyridine-Carboxylate Frameworks.Angew Chem Int Ed Engl. 2024 Jul 22;63(30):e202400823. doi: 10.1002/anie.202400823. Epub 2024 Jun 17. Angew Chem Int Ed Engl. 2024. PMID: 38735839
-
Gate-Opening Effect in a Flexible Metal-Organic Framework for Sieving Acetylene.Chem Bio Eng. 2024 Feb 15;1(2):150-156. doi: 10.1021/cbe.3c00097. eCollection 2024 Mar 28. Chem Bio Eng. 2024. PMID: 39975640 Free PMC article.
-
An Adsorbate Discriminatory Gate Effect in a Flexible Porous Coordination Polymer for Selective Adsorption of CO2 over C2H2.J Am Chem Soc. 2016 Mar 9;138(9):3022-30. doi: 10.1021/jacs.5b10491. Epub 2016 Feb 29. J Am Chem Soc. 2016. PMID: 26876504
-
Deciphering Mechanisms of CO2-Selective Recognition over Acetylene within Porous Materials.Chem Bio Eng. 2024 May 13;1(5):366-380. doi: 10.1021/cbe.4c00035. eCollection 2024 Jun 27. Chem Bio Eng. 2024. PMID: 39975798 Free PMC article. Review.
References
-
- Stang P. J. and Diederich F., Modern acetylene chemistry, John Wiley & Sons, 2008
-
- Reid C. R. Thomas K. M. Langmuir. 1999;15:3206–3218. doi: 10.1021/la981289p. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

