Optoelectronic insights of lead-free layered halide perovskites
- PMID: 38784758
- PMCID: PMC11110173
- DOI: 10.1039/d4sc01429d
Optoelectronic insights of lead-free layered halide perovskites
Abstract
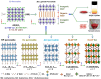
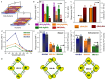
Two-dimensional organic-inorganic halide perovskites have emerged as promising candidates for a multitude of optoelectronic technologies, owing to their versatile structure and electronic properties. The optical and electronic properties are harmoniously integrated with both the inorganic metal halide octahedral slab, and the organic spacer layer. The inorganic octahedral layers can also assemble into periodically stacked nanoplatelets, which are interconnected by the organic ammonium cation, resulting in the formation of a superlattice or superstructure. In this perspective, we explore the structural, electronic, and optical properties of lead-free hybrid halides, and the layered halide perovskite single crystals and nanostructures, expanding our understanding of the diverse applications enabled by these versatile structures. The optical properties of the layered halide perovskite single crystals and superlattices are a function of the organic spacer layer thickness, the metal center with either divalent or a combination of monovalent and trivalent cations, and the halide composition. The distinct absorption and emission features are guided by the structural deformation, electron-phonon coupling, and the polaronic effect. Among the diverse optoelectronic possibilities, we have focused on the photodetection capability of layered halide perovskite single crystals, and elucidated the descriptors such as excitonic band gap, effective mass, carrier mobility, Rashba splitting, and the spin texture that decides the direct component of the optical transitions.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures








Similar articles
-
Tuning Electronic Structure in Layered Hybrid Perovskites with Organic Spacer Substitution.Nano Lett. 2019 Dec 11;19(12):8732-8740. doi: 10.1021/acs.nanolett.9b03427. Epub 2019 Nov 8. Nano Lett. 2019. PMID: 31675242
-
Layered Hybrid Formamidinium Lead Iodide Perovskites: Challenges and Opportunities.Acc Chem Res. 2021 Jun 15;54(12):2729-2740. doi: 10.1021/acs.accounts.0c00879. Epub 2021 Jun 4. Acc Chem Res. 2021. PMID: 34085817
-
Progress and challenges in layered two-dimensional hybrid perovskites.Nanotechnology. 2022 Apr 29;33(29). doi: 10.1088/1361-6528/ac6529. Nanotechnology. 2022. PMID: 35390776
-
A-Site Cation Chemistry in Halide Perovskites.Chem Mater. 2024 Oct 23;36(21):10408-10420. doi: 10.1021/acs.chemmater.4c02043. eCollection 2024 Nov 12. Chem Mater. 2024. PMID: 39554283 Free PMC article. Review.
-
Organic/Inorganic Metal Halide Perovskite Optoelectronic Devices beyond Solar Cells.Adv Sci (Weinh). 2018 Mar 6;5(5):1700780. doi: 10.1002/advs.201700780. eCollection 2018 May. Adv Sci (Weinh). 2018. PMID: 29876207 Free PMC article. Review.
References
-
- NREL, Best Research-Cell Efficiency Chart, Photovoltaic Research, NREL, 2022, https://www.nrel.gov/pv/cellefficiency.html
-
- Zheng C. Rubel O. J. Phys. Chem. C. 2019;123:19385–19394. doi: 10.1021/acs.jpcc.9b05516. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

