Immune response of COVID-19 vaccines in solid cancer patients: A meta-analysis
- PMID: 38785118
- PMCID: PMC11135846
- DOI: 10.1080/21645515.2024.2357424
Immune response of COVID-19 vaccines in solid cancer patients: A meta-analysis
Abstract
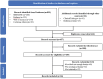
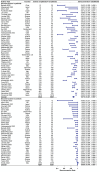
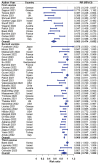
Solid cancer patients, compared to their healthy counterparts, are at a greater risk of contracting and suffering from severe complications and poorer prognosis after COVID-19 infections. They also have different immune responses after doses of COVID-19 vaccination, but limited evidence is available to reveal the effectiveness and help to guide immunization programs for this subpopulation; MEDLINE, Embase, Web of Science, Cochrane Library databases, and clinicaltrials.gov were used to search literature. The pooled seroconversion rate was calculated using a random-effects model and reported with a 95% confidence interval (CI); The review includes 66 studies containing serological responses after COVID-19 vaccination in 13,050 solid cancer patients and 8550 healthy controls. The pooled seropositive rates after the first dose in patients with solid cancer and healthy controls are 55.2% (95% CI 45.9%-64.5% N = 18) and 90.2% (95% CI 80.9%-96.6% N = 13), respectively. The seropositive rates after the second dose in patients with solid cancer and healthy controls are 87.6% (95% CI 84.1%-90.7% N = 50) and 98.9% (95% CI 97.6%-99.7% N = 35), respectively. The seropositive rates after the third dose in patients with solid cancer and healthy controls are 91.4% (95% CI 85.4%-95.9% N = 21) and 99.8% (95% CI 98.1%-100.0% N = 4), respectively. Subgroup analysis finds that study sample size, timing of antibody testing, and vaccine type have influence on the results; Seroconversion rates after COVID-19 vaccination are significantly lower in patients with solid malignancies, especially after the first dose, then shrinking gradually after the following two vaccinations, indicating that subsequent doses or a booster dose should be considered for the effectiveness of this subpopulation.
Keywords: COVID-19; solid cancer; vaccine.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
References
-
- WHO Coronavirus Disease (COVID-19) Dashboard 2021.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical