Role of lactate and lactate metabolism in liver diseases (Review)
- PMID: 38785162
- PMCID: PMC11188982
- DOI: 10.3892/ijmm.2024.5383
Role of lactate and lactate metabolism in liver diseases (Review)
Abstract
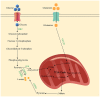
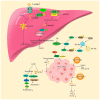
Lactate is a byproduct of glycolysis, and before the Warburg effect was revealed (in which glucose can be fermented in the presence of oxygen to produce lactate) it was considered a metabolic waste product. At present, lactate is not only recognized as a metabolic substrate that provides energy, but also as a signaling molecule that regulates cellular functions under pathophysiological conditions. Lactylation, a post‑translational modification, is involved in the development of various diseases, including inflammation and tumors. Liver disease is a major health challenge worldwide. In normal liver, there is a net lactate uptake caused by gluconeogenesis, exhibiting a higher net lactate clearance rate compared with any other organ. Therefore, abnormalities of lactate and lactate metabolism lead to the development of liver disease, and lactate and lactate metabolism‑related genes can be used for predicting the prognosis of liver disease. Targeting lactate production, regulating lactate transport and modulating lactylation may be potential treatment approaches for liver disease. However, currently there is not a systematic review that summarizes the role of lactate and lactate metabolism in liver diseases. In the present review, the role of lactate and lactate metabolism in liver diseases including liver fibrosis, non‑alcoholic fatty liver disease, acute liver failure and hepatocellular carcinoma was summarized with the aim to provide insights for future research.
Keywords: acute liver failure; hepatocellular carcinoma; lactate; lactate metabolism; non‑alcoholic fatty liver disease.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Souto-Carneiro MM, Klika KD, Abreu MT, Meyer AP, Saffrich R, Sandhoff R, Jennemann R, Kraus FV, Tykocinski L, Eckstein V, et al. Effect of increased lactate dehydrogenase a activity and aerobic glycolysis on the proinflammatory profile of autoimmune CD8+ T cells in rheumatoid arthritis. Arthritis Rheumatol. 2020;72:2050–2064. doi: 10.1002/art.41420. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

