Heterogeneity of comprehensive clinical phenotype and longitudinal adaptive function and correlation with computational predictions of severity of missense genotypes in KIF1A-associated neurological disorder
- PMID: 38785164
- PMCID: PMC11298291
- DOI: 10.1016/j.gim.2024.101169
Heterogeneity of comprehensive clinical phenotype and longitudinal adaptive function and correlation with computational predictions of severity of missense genotypes in KIF1A-associated neurological disorder
Abstract
Purpose: Pathogenic variants in kinesin family member 1A (KIF1A) are associated with KIF1A-associated neurological disorder. We report the clinical phenotypes and correlate genotypes of individuals with KIF1A-associated neurological disorder.
Methods: Medical history and adaptive function were assessed longitudinally. In-person evaluations included neurological, motor, ophthalmologic, and cognitive assessments.
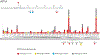
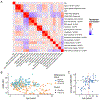
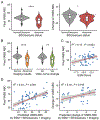
Results: We collected online data on 177 individuals. Fifty-seven individuals were also assessed in-person. Most individuals had de novo heterozygous missense likely pathogenic/pathogenic KIF1A variants. The most common characteristics were hypotonia, spasticity, ataxia, seizures, optic nerve atrophy, cerebellar atrophy, and cognitive impairment. Mean Vineland adaptive behavior composite score (VABS-ABC) was low (M = 62.9, SD = 19.1). The mean change in VABS-ABC over time was -3.1 (SD = 7.3). The decline in VABS-ABC was associated with the age at first assessment and abnormal electroencephalogram/seizure. There was a positive correlation between evolutionary scale model (ESM) score for the variants and final VABS-ABC (P = .003). Abnormal electroencephalogram/seizure, neuroimaging result, and ESM explain 34% of the variance in final VABS-ABC (P < .001).
Conclusion: In-person assessment confirmed caregiver report and identified additional visual deficits. Adaptive function declined over time consistent with both the neurodevelopmental and neurodegenerative nature of the condition. Using ESM score assists in predicting phenotype across a wide range of unique variants.
Keywords: Adaptive function; Cognitive impairment; ESM; KIF1A; Neurological disorder.
Copyright © 2024 American College of Medical Genetics and Genomics. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of Interest The authors declare no conflicts of interest.
Figures

References
-
- Anazawa Y, Kita T, Iguchi R, Hayashi K, Niwa S. De novo mutations in KIF1A-associated neuronal disorder (KAND) dominant-negatively inhibit motor activity and axonal transport of synaptic vesicle precursors. Proc Natl Acad Sci U S A. Aug 9 2022;119(32):e2113795119. doi: 10.1073/pnas.2113795119 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

