12-Month Trajectories of Health-Related Quality of Life Following Hospitalization in German Cancer Centers-A Secondary Data Analysis
- PMID: 38785458
- PMCID: PMC11120277
- DOI: 10.3390/curroncol31050177
12-Month Trajectories of Health-Related Quality of Life Following Hospitalization in German Cancer Centers-A Secondary Data Analysis
Abstract
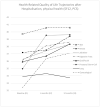
Patient-reported outcomes (PROs) offer a diverse array of potential applications within medical research and clinical practice. In comparative research, they can serve as tools for delineating the trajectories of health-related quality of life (HRQoL) across various cancer types. We undertook a secondary data analysis of a cohort of 1498 hospitalized cancer patients from 13 German cancer centers. We assessed the Physical and Mental Component Scores (PCS and MCS) of the 12-Item Short-Form Health Survey at baseline (t0), 6 (t1), and 12 months (t2), using multivariable generalized linear regression models. At baseline, the mean PCS and MCS values for all cancer patients were 37.1 and 44.3 points, respectively. We observed a significant improvement in PCS at t2 and in MCS at t1. The most substantial and significant improvements were noted among patients with gynecological cancers. We found a number of significant differences between cancer types at baseline, t1, and t2, with skin cancer patients performing best across all time points and lung cancer patients performing the worst. MCS trajectories showed less pronounced changes and differences between cancer types. Comparative analyses of HRQoL scores across different cancer types may serve as a valuable tool for enhancing health literacy, both among the general public and among cancer patients themselves.
Keywords: SF12; health literacy; health-related quality of life; prospective longitudinal study.
Conflict of interest statement
The authors have no relevant financial or non-financial interests to disclose.
Figures


References
-
- U.S. Department of Health and Human Services FDA Center for Drug Evaluation and Research. U.S. Department of Health and Human Services FDA Center for Biologics Evaluation and Research. U.S. Department of Health and Human Services FDA Center for Devices and Radiological Health Guidance for Industry Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims: Draft Guidance. Health Qual. Life Outcomes. 2006;4:79. doi: 10.1186/1477-7525-4-79. - DOI - PMC - PubMed
-
- Kulis D., Bottomley A., Whittaker C., van de Poll-Franse L., Darlington A., Holzner B., Koller M., Reijneveld J., Tomaszewski K., Grønvold M. The Use of the Eortc Item Library to Supplement Eortc Quality of Life Instruments. Value Health. 2017;20:A775. doi: 10.1016/j.jval.2017.08.2236. - DOI
-
- Webster K.A., O’Connor M.L., Hansen A.R., Kircher S., Jim H.S.L., Dicker A.P., Janda M., Ala-leppilampi K., Bingham C.O., Feliciano J., et al. Development of a Functional Assessment of Chronic Illness Therapy Item Library and Primary Symptom List for the Assessment of Patient-Reported Adverse Events Associated with Immune Checkpoint Modulators. J. Cancer Metastasis Treat. 2020;6:8. doi: 10.20517/2394-4722.2019.38. - DOI - PMC - PubMed
-
- Hinz A., Weis J., Faller H., Brähler E., Härter M., Keller M., Schulz H., Wegscheider K., Koch U., Geue K., et al. Quality of Life in Cancer Patients-a Comparison of Inpatient, Outpatient, and Rehabilitation Settings. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer. 2018;26:3533–3541. doi: 10.1007/s00520-018-4211-4. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

