Insights into the Activation of Unfolded Protein Response Mechanism during Coronavirus Infection
- PMID: 38785529
- PMCID: PMC11120126
- DOI: 10.3390/cimb46050261
Insights into the Activation of Unfolded Protein Response Mechanism during Coronavirus Infection
Abstract
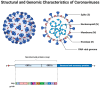
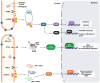
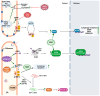
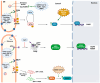
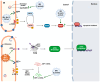
Coronaviruses represent a significant class of viruses that affect both animals and humans. Their replication cycle is strongly associated with the endoplasmic reticulum (ER), which, upon virus invasion, triggers ER stress responses. The activation of the unfolded protein response (UPR) within infected cells is performed from three transmembrane receptors, IRE1, PERK, and ATF6, and results in a reduction in protein production, a boost in the ER's ability to fold proteins properly, and the initiation of ER-associated degradation (ERAD) to remove misfolded or unfolded proteins. However, in cases of prolonged and severe ER stress, the UPR can also instigate apoptotic cell death and inflammation. Herein, we discuss the ER-triggered host responses after coronavirus infection, as well as the pharmaceutical targeting of the UPR as a potential antiviral strategy.
Keywords: ATF6; CoV; ER stress; IRE1; PERK; coronavirus; pharmacological inhibition; unfolded protein response.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
The PERK Arm of the Unfolded Protein Response Negatively Regulates Transmissible Gastroenteritis Virus Replication by Suppressing Protein Translation and Promoting Type I Interferon Production.J Virol. 2018 Jul 17;92(15):e00431-18. doi: 10.1128/JVI.00431-18. Print 2018 Aug 1. J Virol. 2018. PMID: 29769338 Free PMC article.
-
The Human Cytomegalovirus Endoplasmic Reticulum-Resident Glycoprotein UL148 Activates the Unfolded Protein Response.J Virol. 2018 Sep 26;92(20):e00896-18. doi: 10.1128/JVI.00896-18. Print 2018 Oct 15. J Virol. 2018. PMID: 30045994 Free PMC article.
-
KSHV activates unfolded protein response sensors but suppresses downstream transcriptional responses to support lytic replication.PLoS Pathog. 2019 Dec 2;15(12):e1008185. doi: 10.1371/journal.ppat.1008185. eCollection 2019 Dec. PLoS Pathog. 2019. PMID: 31790507 Free PMC article.
-
Coronavirus-induced ER stress response and its involvement in regulation of coronavirus-host interactions.Virus Res. 2014 Dec 19;194:110-23. doi: 10.1016/j.virusres.2014.09.016. Epub 2014 Oct 7. Virus Res. 2014. PMID: 25304691 Free PMC article. Review.
-
The roles and mechanisms of endoplasmic reticulum stress-mediated autophagy in animal viral infections.Vet Res. 2024 Sep 3;55(1):107. doi: 10.1186/s13567-024-01360-4. Vet Res. 2024. PMID: 39227990 Free PMC article. Review.
Cited by
-
Acute COVID-19 and LongCOVID syndrome - molecular implications for therapeutic strategies - review.Front Immunol. 2025 Apr 17;16:1582783. doi: 10.3389/fimmu.2025.1582783. eCollection 2025. Front Immunol. 2025. PMID: 40313948 Free PMC article. Review.
-
Spring viraemia of carp virus modulates the time-dependent unfolded protein response to facilitate viral replication.Front Immunol. 2025 Apr 3;16:1576758. doi: 10.3389/fimmu.2025.1576758. eCollection 2025. Front Immunol. 2025. PMID: 40248709 Free PMC article.
-
Bibliometric analysis of autophagy in NAFLD from 2004 to 2023.Medicine (Baltimore). 2024 Dec 6;103(49):e40835. doi: 10.1097/MD.0000000000040835. Medicine (Baltimore). 2024. PMID: 39654183 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources

