Polyhexamethylene Biguanide Reduces High-Risk Human Papilloma Virus Viral Load in Cervical Cell Samples Derived from ThinPrep Pap Test
- PMID: 38785561
- PMCID: PMC11119563
- DOI: 10.3390/cimb46050293
Polyhexamethylene Biguanide Reduces High-Risk Human Papilloma Virus Viral Load in Cervical Cell Samples Derived from ThinPrep Pap Test
Abstract
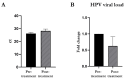
Human papilloma virus (HPV) infection and its progression still represent a great medical challenge worldwide. Clinical evidence has demonstrated the beneficial effects of polyhexamethylene biguanide (PHMB) on HPV clinical manifestations; however, evidence of the effect of this molecule on HPV viral load is still lacking. In this in vitro study, 13 ThinPrep Papanicolaou (Pap) tests were treated with a PHMB solution (0.10 g/100 mL) for 2 h. We observed no cytological changes but a significant reduction in the viral load of high-risk (HR) HPV after PHMB treatment, also revealing a dose-dependent antiviral effect. In addition, by stratifying the obtained results according to HR-HPV genotype, we observed a significant reduction in the viral load of HPV 16, P2 (56, 59, 66), 31, and P3 (35, 39, 68) and a strong decrease in the viral load of HPV 45, 52, and P1 (33, 58). Overall, 85% of the analyzed cervical cell samples exhibited an improvement in HPV viral load after PHMB exposure, while only 15% remain unchanged. For the first time, the data from this pilot study support the activity of PHMB on a specific phase of the HPV viral lifecycle, the one regarding the newly generated virions, reducing viral load and thus blocking the infection of other cervical cells.
Keywords: HPV; PHMB; ThinPrep Pap test; cervical cell samples; viral load.
Conflict of interest statement
S.P. and E.L. are employed by Lo.Li. Pharma Srl, Rome (Italy). The other authors declare no conflicts of interest.
Figures



Similar articles
-
Is p16(INK4A) expression more useful than human papillomavirus test to determine the outcome of atypical squamous cells of undetermined significance-categorized Pap smear? A comparative analysis using abnormal cervical smears with follow-up biopsies.Gynecol Oncol. 2005 Apr;97(1):35-40. doi: 10.1016/j.ygyno.2004.11.034. Gynecol Oncol. 2005. PMID: 15790434
-
Maximizing the adequacy of Hologic(®) Cervista(®) HPV HR results on ThinPrep(®) Pap samples treated with glacial acetic acid.Diagn Cytopathol. 2016 Mar;44(3):215-9. doi: 10.1002/dc.23426. Epub 2016 Jan 18. Diagn Cytopathol. 2016. PMID: 26779984
-
Which test is a better strategy to determine the outcome of atypical glandular cell-categorized Pap smears? Immunocytochemical p16INK4A expression or human papillomavirus test--a retrospective cohort study.Gynecol Oncol. 2005 Dec;99(3):578-84. doi: 10.1016/j.ygyno.2005.06.060. Epub 2005 Sep 1. Gynecol Oncol. 2005. PMID: 16139346
-
A new non-invasive approach based on polyhexamethylene biguanide increases the regression rate of HPV infection.BMC Clin Pathol. 2012 Sep 25;12:17. doi: 10.1186/1472-6890-12-17. BMC Clin Pathol. 2012. PMID: 23009652 Free PMC article.
-
Negative computer-imaged ThinPrep Pap test and positive hybrid capture2 HPV co-testing results: A quality assurance review.Diagn Cytopathol. 2015 Sep;43(9):763-9. doi: 10.1002/dc.23303. Epub 2015 Jul 15. Diagn Cytopathol. 2015. PMID: 26173579 Review.
References
-
- Liverani C.A. The four steps in the prevention of human papillomavirus-associated neoplasia: Considerations for preventive measures, screening, disease impact, and potential overtreatments in HPV-related pathology. Arch. Gynecol. Obstet. 2013;288:979–988. doi: 10.1007/s00404-013-3011-9. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

