Comparing Redox and Intracellular Signalling Responses to Cold Plasma in Wound Healing and Cancer
- PMID: 38785562
- PMCID: PMC11120013
- DOI: 10.3390/cimb46050294
Comparing Redox and Intracellular Signalling Responses to Cold Plasma in Wound Healing and Cancer
Abstract
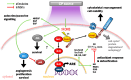
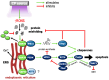
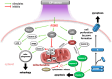
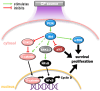
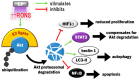
Cold plasma (CP) is an ionised gas containing excited molecules and ions, radicals, and free electrons, and which emits electric fields and UV radiation. CP is potently antimicrobial, and can be applied safely to biological tissue, birthing the field of plasma medicine. Reactive oxygen and nitrogen species (RONS) produced by CP affect biological processes directly or indirectly via the modification of cellular lipids, proteins, DNA, and intracellular signalling pathways. CP can be applied at lower levels for oxidative eustress to activate cell proliferation, motility, migration, and antioxidant production in normal cells, mainly potentiated by the unfolded protein response, the nuclear factor-erythroid factor 2-related factor 2 (Nrf2)-activated antioxidant response element, and the phosphoinositide 3-kinase/protein kinase B (PI3K/Akt) pathway, which also activates nuclear factor-kappa B (NFκB). At higher CP exposures, inactivation, apoptosis, and autophagy of malignant cells can occur via the degradation of the PI3K/Akt and mitogen-activated protein kinase (MAPK)-dependent and -independent activation of the master tumour suppressor p53, leading to caspase-mediated cell death. These opposing responses validate a hormesis approach to plasma medicine. Clinical applications of CP are becoming increasingly realised in wound healing, while clinical effectiveness in tumours is currently coming to light. This review will outline advances in plasma medicine and compare the main redox and intracellular signalling responses to CP in wound healing and cancer.
Keywords: MAPK; Nrf2; PI3K/Akt; cancer; cold plasma; endoplasmic reticulum stress; plasma-activated water; redox signalling; wound healing.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
ROS and RNS signalling: adaptive redox switches through oxidative/nitrosative protein modifications.Free Radic Res. 2018 May;52(5):507-543. doi: 10.1080/10715762.2018.1457217. Epub 2018 Apr 19. Free Radic Res. 2018. PMID: 29589770 Review.
-
Xihuang pills induce apoptosis in hepatocellular carcinoma by suppressing phosphoinositide 3-kinase/protein kinase-B/mechanistic target of rapamycin pathway.World J Gastrointest Oncol. 2022 Apr 15;14(4):872-886. doi: 10.4251/wjgo.v14.i4.872. World J Gastrointest Oncol. 2022. PMID: 35582102 Free PMC article.
-
Altersolanol B, a fungal tetrahydroanthraquinone, inhibits the proliferation of estrogen receptor-expressing (ER+) human breast adenocarcinoma by modulating PI3K/AKT, p38/ERK MAPK and associated signaling pathways.Chem Biol Interact. 2022 May 25;359:109916. doi: 10.1016/j.cbi.2022.109916. Epub 2022 Mar 26. Chem Biol Interact. 2022. PMID: 35346647
-
Nrf2 signaling and inflammation are key events in physical plasma-spurred wound healing.Theranostics. 2019 Jan 30;9(4):1066-1084. doi: 10.7150/thno.29754. eCollection 2019. Theranostics. 2019. PMID: 30867816 Free PMC article.
-
Redox for Repair: Cold Physical Plasmas and Nrf2 Signaling Promoting Wound Healing.Antioxidants (Basel). 2018 Oct 19;7(10):146. doi: 10.3390/antiox7100146. Antioxidants (Basel). 2018. PMID: 30347767 Free PMC article. Review.
Cited by
-
Plasma-activated water accelerates wound healing and reduces Staphylococcus aureus infection in vivo.Biofilm. 2025 Aug 5;10:100308. doi: 10.1016/j.bioflm.2025.100308. eCollection 2025 Dec. Biofilm. 2025. PMID: 40823344 Free PMC article.
-
[Research advances on the mechanism and clinical application of cold atmospheric plasma in promoting wound healing].Zhonghua Shao Shang Yu Chuang Mian Xiu Fu Za Zhi. 2025 Jun 20;41(6):604-608. doi: 10.3760/cma.j.cn501225-20241117-00448. Zhonghua Shao Shang Yu Chuang Mian Xiu Fu Za Zhi. 2025. PMID: 40588409 Free PMC article. Review. Chinese.
-
Cold Atmospheric Plasma Enhances TGF-β1, CTGF Protein Expression, and Healing in Full-Thickness Skin Burns: An Animal Study.Biomolecules. 2025 Jun 24;15(7):924. doi: 10.3390/biom15070924. Biomolecules. 2025. PMID: 40723796 Free PMC article.
-
Fluidic-manipulation-enabled multiplexed dose delivery of RONS by a CAP chip for dose optimization enhancement.Microsyst Nanoeng. 2025 Jun 16;11(1):123. doi: 10.1038/s41378-025-00974-8. Microsyst Nanoeng. 2025. PMID: 40518485 Free PMC article.
References
-
- Tendero C., Tixier C., Tristant P., Desmaison J., Leprince P. Atmospheric pressure plasmas: A review. Spectrochim. Acta Part B Spectrosc. 2006;61:2–30. doi: 10.1016/j.sab.2005.10.003. - DOI
-
- von Woedtke T., Laroussi M., Gherardi M. Foundations of plasmas for medical applications. Plasma Sources Sci. Technol. 2022;31:39. doi: 10.1088/1361-6595/ac604f. - DOI
-
- von Woedtke T., Emmert S., Metelmann H.-R., Rupf S., Weltmann K.-D. Perspectives on cold atmospheric plasma (CAP) applications in medicine. Phys. Plasmas. 2020;27:070601. doi: 10.1063/5.0008093. - DOI
-
- Brandenburg R. Dielectric barrier discharges: Progress on plasma sources and on the understanding of regimes and single filaments. Plasma Sources Sci. Technol. 2017;26:053001. doi: 10.1088/1361-6595/aa6426. - DOI
-
- van Welzen A., Hoch M., Wahl P., Weber F., Rode S., Tietze J.K., Boeckmann L., Emmert S., Thiem A. The Response and Tolerability of a Novel Cold Atmospheric Plasma Wound Dressing for the Healing of Split Skin Graft Donor Sites: A Controlled Pilot Study. Ski. Pharmacol. Physiol. 2021;34:328–336. doi: 10.1159/000517524. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

