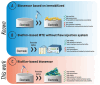
Targeted Formation of Biofilms on the Surface of Graphite Electrodes as an Effective Approach to the Development of Biosensors for Early Warning Systems
- PMID: 38785713
- PMCID: PMC11118945
- DOI: 10.3390/bios14050239
Targeted Formation of Biofilms on the Surface of Graphite Electrodes as an Effective Approach to the Development of Biosensors for Early Warning Systems
Abstract
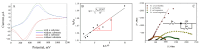
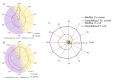
Biofilms based on bacteria Pseudomonas veronii (Ps. veronii) and Escherichia coli (E. coli) and yeast Saccharomyces cerevisiae (S. cerevisiae) were used for novel biosensor creation for rapid biochemical oxygen demand (BOD) monitoring. Based on the electrochemical measurement results, it was shown that the endogenous mediator in the matrix of E. coli and Ps. veronii biofilms and ferrocene form a two-mediator system that improves electron transport in the system. Biofilms based on Ps. veronii and E. coli had a high biotechnological potential for BOD assessment; bioreceptors based on such biofilms had high sensitivity (the lower limits of detectable BOD5 concentrations were 0.61 (Ps. veronii) and 0.87 (E. coli) mg/dm3) and high efficiency of analysis (a measurement time 5-10 min). The maximum biosensor response based on bacterial biofilms has been observed in the pH range of 6.6-7.2. The greatest protective effect was found for biofilms based on E. coli, which has high long-term stability (151 days for Ps. veronii and 163 days for E. coli). The results of the BOD5 analysis of water samples obtained using the developed biosensors had a high correlation with the results of the standard 5-day method (R2 = 0.9820, number of tested samples is 10 for Ps. veronii, and R2 = 0.9862, number of tested samples is 10 for E. coli). Thus, biosensors based on Ps. veronii biofilms and E. coli biofilms could be a novel analytical system to give early warnings of pollution.
Keywords: BOD biosensors; Escherichia coli; Pseudomonas veronii; Saccharomyces cerevisiae; biofilms.
Conflict of interest statement
Authors Ivan Saltanov and Sergey Melenkov were employed by the company “INNOBIOSYSTEMS”. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- ISO Water Quality: Determination of Biochemical Oxygen Demand after n Days (BODn), Part 1: Dilution and Seeding Method with Allylthiourea Addition. International Organization for Standardization; Geneva, Switzerland: 2003.
-
- ISO Water Quality: Determination of Biochemical Oxygen Demand after n Days (BODn), Part 2: Dilution and Seeding Method with Allylthiourea Addition. International Organization for Standardization; Geneva, Switzerland: 2003.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

