Evaluation of the Safety and Efficacy of Repeated Mesenchymal Stem Cell Transplantations in ALS Patients by Investigating Patients' Specific Immunological and Biochemical Biomarkers
- PMID: 38785754
- PMCID: PMC11120501
- DOI: 10.3390/diseases12050099
Evaluation of the Safety and Efficacy of Repeated Mesenchymal Stem Cell Transplantations in ALS Patients by Investigating Patients' Specific Immunological and Biochemical Biomarkers
Abstract
Background: Amyotrophic lateral sclerosis (ALS) is an incurable disease. There are vigorous attempts to develop treatments to reduce the effects of this disease, and among these treatments is the transplantation of stem cells. This study aimed to retrospectively evaluate a mesenchymal stem cell (MSC) therapy cohort as a promising novel treatment modality by estimating some additional new parameters, such as immunological and biochemical factors.
Methods: This study was designed as an open-label, one-arm cohort retrospective study to evaluate potential diagnostic biomarkers of repeated infusions of autologous-bone marrow-derived mesenchymal stem cells (BM-MSCs) in 15 confirmed patients with ALS, administered at a dose of 1 × 106 cells/kg BW with a one-month interval, in equal amounts in both an intravenous (IV) and intrathecal (IT) capacity simultaneously, via various biochemical (iron (Fe), ferritin, total-iron-binding capacity (TIBC), transferrin, and creatine kinase (CK)) and immunological parameters (tumor necrosis factor-alpha (TNF-α), neurofilament light chain (NFL), and glial-cell-derived neurotrophic factor (GDNF) levels, evaluated during the three-month follow-up period in serum and cerebrospinal fluid (CSF).
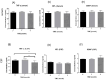
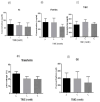
Results: Our study indicated that, in the case of immunological biomarkers, TNF-α levels in the CSF showed a significant decrease at month three after transplantation compared with levels at month zero, and the p-value was p < 0.01. No statistically significant changes were observed for other immunological as well as biochemical parameters and a p-value of p > 0.05.
Conclusions: These results can indicate the potential benefit of stem cell transfusion in patients with ALS and suggest some diagnostic biomarkers. Several studies are required to approve these results.
Keywords: ALS; BM-MSC; GDNF; creatine kinase; ferritin; tumor necrosis factor-alpha.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Evaluation of Safety and Efficacy of Repeated Mesenchymal Stem Cell Transplantation in Patients with Amyotrophic Lateral Sclerosis (ALS) by Investigating Patient's Specific microRNAs as Novel Biomarkers: A Clinical Trial Study.Curr Stem Cell Res Ther. 2025 Jan 29. doi: 10.2174/011574888X330199250106081717. Online ahead of print. Curr Stem Cell Res Ther. 2025. PMID: 39886777 Clinical Trial.
-
Phase I trial of repeated intrathecal autologous bone marrow-derived mesenchymal stromal cells in amyotrophic lateral sclerosis.Stem Cells Transl Med. 2015 Jun;4(6):590-7. doi: 10.5966/sctm.2014-0212. Epub 2015 May 1. Stem Cells Transl Med. 2015. PMID: 25934946 Free PMC article. Clinical Trial.
-
Safety and efficacy of bone marrow derived-mesenchymal stem cells transplantation in patients with amyotrophic lateral sclerosis.Regen Ther. 2021 Aug 16;18:268-274. doi: 10.1016/j.reth.2021.07.006. eCollection 2021 Dec. Regen Ther. 2021. PMID: 34466632 Free PMC article.
-
Neurofilament Light Chain as Biomarker for Amyotrophic Lateral Sclerosis and Frontotemporal Dementia.Front Neurosci. 2021 Jun 21;15:679199. doi: 10.3389/fnins.2021.679199. eCollection 2021. Front Neurosci. 2021. PMID: 34234641 Free PMC article. Review.
-
Aberrations of biochemical indicators in amyotrophic lateral sclerosis: a systematic review and meta-analysis.Transl Neurodegener. 2021 Jan 8;10(1):3. doi: 10.1186/s40035-020-00228-9. Transl Neurodegener. 2021. PMID: 33419478 Free PMC article.
References
-
- ALS Managed Care ConsiderationsSantaniello, Briana. Am. J. Manag. Care. 2018;24:S336–S341. - PubMed
-
- Kwiatkowski Jr T.J., Bosco D.A., Leclerc A.L., Tamrazian E., Vanderburg C.R., Russ C., Davis A., Gilchrist J., Kasarskis E.J., Munsat T. Mutations in the FUS/TLS Gene on Chromosome 16 Cause Familial Amyotrophic Lateral Sclerosis. Science. 2009;323:1205–1208. doi: 10.1126/science.1166066. - DOI - PubMed
-
- DeJesus-Hernandez M., Mackenzie I.R., Boeve B.F., Boxer A.L., Baker M., Rutherford N.J., Nicholson A.M., Finch N.A., Flynn H., Adamson J. Expanded GGGGCC Hexanucleotide Repeat in Noncoding Region of C9ORF72 Causes Chromosome 9p-Linked FTD and ALS. Neuron. 2011;72:245–256. doi: 10.1016/j.neuron.2011.09.011. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

