Potential Role of Pig UCP3 in Modulating Adipocyte Browning via the Beta-Adrenergic Receptor Signaling Pathway
- PMID: 38785767
- PMCID: PMC11117546
- DOI: 10.3390/biology13050284
Potential Role of Pig UCP3 in Modulating Adipocyte Browning via the Beta-Adrenergic Receptor Signaling Pathway
Abstract
Adipose tissue plays an important role in regulating body temperature and metabolism, with white adipocytes serving as storage units for energy. Recent research focused on the browning of white adipocytes (beige adipocytes), causing thermogenesis and lipolysis. The process of browning is linked to the activation of uncoupling protein (UCP) expression, which can be mediated by the β3 adrenergic receptor pathway. Transcriptional factors, such as peroxisome proliferator activated receptor γ (PPARγ) and PPARγ coactivator 1 alpha, play vital roles in cell fate determination for fat cells. Beige adipocytes have metabolic therapeutic potential to combat diseases such as obesity, diabetes mellitus, and dyslipidemia, owing to their significant impact on metabolic functions. However, the molecular mechanisms that cause the induction of browning are unclear. Therefore, research using animal models and primary culture is essential to provide an understanding of browning for further application in human metabolic studies. Pigs have physiological similarities to humans; hence, they are valuable models for research on adipose tissue. This study demonstrates the browning potential of pig white adipocytes through primary culture experiments. The results show that upregulation of UCP3 gene expression and fragmentation of lipid droplets into smaller particles occur due to isoproterenol stimulation, which activates beta-adrenergic receptor signaling. Furthermore, PPARγ and PGC-1α were found to activate the UCP3 promoter region, similar to that of UCP1. These findings suggest that pigs undergo metabolic changes that induce browning in white adipocytes, providing a promising approach for metabolic research with potential implications for human health. This study offers valuable insights into the mechanism of adipocyte browning using pig primary culture that can enhance our understanding of human metabolism, leading to cures for commonly occurring diseases.
Keywords: adipocyte; animal model; browning; fat primary culture; lipolysis; uncoupling protein.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

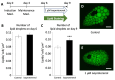
 ) control, (
) control, ( ) 0.01 µM, (
) 0.01 µM, ( ) 0.1 µM, (
) 0.1 µM, ( ) 1 µM, (
) 1 µM, ( ) 10 µM, and (
) 10 µM, and ( ) 100 µM. The values are shown as mean ± SEM. * p < 0.05 and ** p < 0.01.
) 100 µM. The values are shown as mean ± SEM. * p < 0.05 and ** p < 0.01.



References
Grants and funding
LinkOut - more resources
Full Text Sources

