The Influence of Stress and Binge-Patterned Alcohol Drinking on Mouse Skeletal Muscle Protein Synthesis and Degradation Pathways
- PMID: 38785934
- PMCID: PMC11118922
- DOI: 10.3390/biom14050527
The Influence of Stress and Binge-Patterned Alcohol Drinking on Mouse Skeletal Muscle Protein Synthesis and Degradation Pathways
Abstract
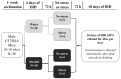
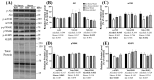
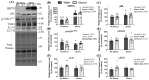
Adverse experiences (e.g., acute stress) and alcohol misuse can both impair skeletal muscle homeostasis, resulting in reduced protein synthesis and greater protein breakdown. Exposure to acute stress is a significant risk factor for engaging in alcohol misuse. However, little is known about how these factors together might further affect skeletal muscle health. To that end, this study investigated the effects of acute stress exposure followed by a period of binge-patterned alcohol drinking on signaling factors along mouse skeletal muscle protein synthesis (MPS) and degradation (MPD) pathways. Young adult male C57BL/6J mice participated in the Drinking in the Dark paradigm, where they received 2-4 h of access to 20% ethanol (alcohol group) or water (control group) for four days to establish baseline drinking levels. Three days later, half of the mice in each group were either exposed to a single episode of uncontrollable tail shocks (acute stress) or remained undisturbed in their home cages (no stress). Three days after stress exposure, mice received 4 h of access to 20% ethanol (alcohol) to model binge-patterned alcohol drinking or water for ten consecutive days. Immediately following the final episode of alcohol access, mouse gastrocnemius muscle was extracted to measure changes in relative protein levels along the Akt-mTOR MPS, as well as the ubiquitin-proteasome pathway (UPP) and autophagy MPD pathways via Western blotting. A single exposure to acute stress impaired Akt singling and reduced rates of MPS, independent of alcohol access. This observation was concurrent with a potent increase in heat shock protein seventy expression in the muscle of stressed mice. Alcohol drinking did not exacerbate stress-induced alterations in the MPS and MPD signaling pathways. Instead, changes in the MPS and MPD signaling factors due to alcohol access were primarily observed in non-stressed mice. Taken together, these data suggest that exposure to a stressor of sufficient intensity may cause prolonged disruptions to signaling factors that impact skeletal muscle health and function beyond what could be further induced by periods of alcohol misuse.
Keywords: autophagy; binge-patterned drinking; drinking in the dark; muscle protein degradation; muscle protein synthesis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
The effects of voluntary binge-patterned ethanol ingestion and daily wheel running on signaling of muscle protein synthesis and degradation in female mice.Alcohol. 2022 Nov;104:45-52. doi: 10.1016/j.alcohol.2022.06.004. Epub 2022 Aug 2. Alcohol. 2022. PMID: 35926812
-
Dysregulation of skeletal muscle protein metabolism by alcohol.Am J Physiol Endocrinol Metab. 2015 May 1;308(9):E699-712. doi: 10.1152/ajpendo.00006.2015. Epub 2015 Mar 10. Am J Physiol Endocrinol Metab. 2015. PMID: 25759394 Free PMC article. Review.
-
Acute Alcohol-Induced Decrease in Muscle Protein Synthesis in Female Mice Is REDD-1 and mTOR-Independent.Alcohol Alcohol. 2016 May;51(3):242-50. doi: 10.1093/alcalc/agv105. Epub 2015 Sep 22. Alcohol Alcohol. 2016. PMID: 26394774 Free PMC article.
-
Ethanol acutely antagonizes the refeeding-induced increase in mTOR-dependent protein synthesis and decrease in autophagy in skeletal muscle.Mol Cell Biochem. 2019 Jun;456(1-2):41-51. doi: 10.1007/s11010-018-3488-4. Epub 2018 Dec 6. Mol Cell Biochem. 2019. PMID: 30523512 Free PMC article.
-
Alcohol impairs skeletal muscle protein synthesis and mTOR signaling in a time-dependent manner following electrically stimulated muscle contraction.J Appl Physiol (1985). 2014 Nov 15;117(10):1170-9. doi: 10.1152/japplphysiol.00180.2014. Epub 2014 Sep 25. J Appl Physiol (1985). 2014. PMID: 25257868 Free PMC article.
References
-
- Kessler R.C., Crum R.M., Warner L.A., Nelson C.B., Schulenberg J., Anthony J.C. Lifetime Co-Occurence of DSM-III-R Alcohol Abuse and Dependence with Other Psychiatric Disorders in the National Comorbidity Study. Arch. Gen. Psychiatry. 1997;54:313–321. doi: 10.1001/archpsyc.1997.01830160031005. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

