Estetrol Inhibits Endometriosis Development in an In Vivo Murine Model
- PMID: 38785987
- PMCID: PMC11118049
- DOI: 10.3390/biom14050580
Estetrol Inhibits Endometriosis Development in an In Vivo Murine Model
Abstract
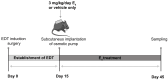
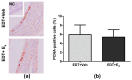
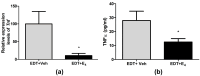
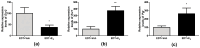
Endometriosis is characterized by the growth of endometrial-like tissue outside the uterus, and it is associated with alterations in the expression of hormone receptors and inflammation. Estetrol (E4) is a weak estrogen that recently has been approved for contraception. We evaluated the effect of E4 on the growth of endometriotic-like lesions and the expression of TNF-α, estrogen receptors (ERs), and progesterone receptors (PRs) in an in vivo murine model. Endometriosis was induced surgically in female C57BL/6 mice. E4 was delivered via Alzet pump (3 mg/kg/day) from the 15th postoperative day for 4 weeks. E4 significantly reduced the volume (p < 0.001) and weight (p < 0.05) of ectopic lesions. Histologically, E4 did not affect cell proliferation (PCNA immunohistochemistry) but it did increase cell apoptosis (TUNEL assay) (p < 0.05). Furthermore, it modulated oxidative stress (SOD, CAT, and GPX activity, p < 0.05) and increased lipid peroxidation (TBARS/MDA, p < 0.01). Molecular analysis showed mRNA (RT-qPCR) and protein (ELISA) expression of TNF-α decreased (p < 0.05) and mRNA expression of Esr2 reduced (p < 0.05), in contrast with the increased expression of Esr1 (p < 0.01) and Pgr (p < 0.05). The present study demonstrates for the first time that E4 limited the development and progression of endometriosis in vivo.
Keywords: apoptosis; endometriosis; estetrol; hormone receptors; mouse model; oxidative stress; proliferation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Huhtinen K., Desai R., Ståhle M., Salminen A., Handelsman D.J., Perheentupa A., Poutanen M. Endometrial and Endometriotic Concentrations of Estrone and Estradiol Are Determined by Local Metabolism Rather than Circulating Levels. J. Clin. Endocrinol. Metab. 2012;97:4228–4235. doi: 10.1210/jc.2012-1154. - DOI - PMC - PubMed
-
- Han S.J., Jung S.Y., Wu S.-P., Hawkins S.M., Park M.J., Kyo S., Qin J., Lydon J.P., Tsai S.Y., Tsai M.-J., et al. Estrogen Receptor β Modulates Apoptosis Complexes and the Inflammasome to Drive the Pathogenesis of Endometriosis. Cell. 2015;163:960–974. doi: 10.1016/j.cell.2015.10.034. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

