In Vitro Investigation of Therapy-Induced Senescence and Senescence Escape in Breast Cancer Cells Using Novel Flow Cytometry-Based Methods
- PMID: 38786063
- PMCID: PMC11120107
- DOI: 10.3390/cells13100841
In Vitro Investigation of Therapy-Induced Senescence and Senescence Escape in Breast Cancer Cells Using Novel Flow Cytometry-Based Methods
Abstract
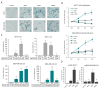
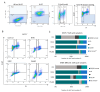
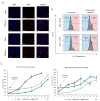
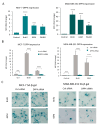
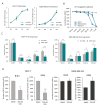
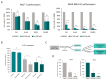
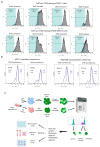
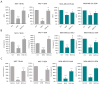
Although cellular senescence was originally defined as an irreversible form of cell cycle arrest, in therapy-induced senescence models, the emergence of proliferative senescence-escaped cancer cells has been reported by several groups, challenging the definition of senescence. Indeed, senescence-escaped cancer cells may contribute to resistance to cancer treatment. Here, to study senescence escape and isolate senescence-escaped cells, we developed novel flow cytometry-based methods using the proliferation marker Ki-67 and CellTrace CFSE live-staining. We investigated the role of a novel senescence marker (DPP4/CD26) and a senolytic drug (azithromycin) on the senescence-escaping ability of MCF-7 and MDA-MB-231 breast cancer cells. Our results show that the expression of DPP4/CD26 is significantly increased in both senescent MCF-7 and MDA-MB-231 cells. While not essential for senescence induction, DPP4/CD26 contributed to promoting senescence escape in MCF-7 cells but not in MDA-MB-231 cells. Our results also confirmed the potential senolytic effect of azithromycin in senescent cancer cells. Importantly, the combination of azithromycin and a DPP4 inhibitor (sitagliptin) demonstrated a synergistic effect in senescent MCF-7 cells and reduced the number of senescence-escaped cells. Although further research is needed, our results and novel methods could contribute to the investigation of the mechanisms of senescence escape and the identification of potential therapeutic targets. Indeed, DPP4/CD26 could be a promising marker and a novel target to potentially decrease senescence escape in cancer.
Keywords: breast cancer; flow cytometry; senescence escape; therapy-induced senescence.
Conflict of interest statement
MPL and FS hold a minority interest in Lunella Biotech, Inc.
Figures

Similar articles
-
CD26 is a senescence marker associated with reduced immunopotency of human adipose tissue-derived multipotent mesenchymal stromal cells.Stem Cell Res Ther. 2022 Jul 26;13(1):358. doi: 10.1186/s13287-022-03026-4. Stem Cell Res Ther. 2022. PMID: 35883188 Free PMC article.
-
Ginsenoside Rh2 Ameliorates Doxorubicin-Induced Senescence Bystander Effect in Breast Carcinoma Cell MDA-MB-231 and Normal Epithelial Cell MCF-10A.Int J Mol Sci. 2019 Mar 12;20(5):1244. doi: 10.3390/ijms20051244. Int J Mol Sci. 2019. PMID: 30871042 Free PMC article.
-
Improved Autophagic Flux in Escapers from Doxorubicin-Induced Senescence/Polyploidy of Breast Cancer Cells.Int J Mol Sci. 2020 Aug 24;21(17):6084. doi: 10.3390/ijms21176084. Int J Mol Sci. 2020. PMID: 32846959 Free PMC article.
-
CD26/DPP4 - a potential biomarker and target for cancer therapy.Pharmacol Ther. 2019 Jun;198:135-159. doi: 10.1016/j.pharmthera.2019.02.015. Epub 2019 Feb 26. Pharmacol Ther. 2019. PMID: 30822465 Review.
-
Cellular senescence: from anti-cancer weapon to anti-aging target.Sci China Life Sci. 2020 Mar;63(3):332-342. doi: 10.1007/s11427-019-1629-6. Epub 2020 Feb 13. Sci China Life Sci. 2020. PMID: 32060861 Review.
Cited by
-
Senescence-related genes as prognostic indicators in breast cancer survival.Geroscience. 2025 Jun;47(3):2995-3006. doi: 10.1007/s11357-024-01384-w. Epub 2024 Oct 21. Geroscience. 2025. PMID: 39432147 Free PMC article.
-
Therapy-induced senescence is a transient drug resistance mechanism in breast cancer.Mol Cancer. 2025 May 1;24(1):128. doi: 10.1186/s12943-025-02310-0. Mol Cancer. 2025. PMID: 40312750 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

