Immune Cell Migration to Cancer
- PMID: 38786066
- PMCID: PMC11120175
- DOI: 10.3390/cells13100844
Immune Cell Migration to Cancer
Abstract
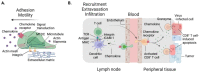
Immune cell migration is required for the development of an effective and robust immune response. This elegant process is regulated by both cellular and environmental factors, with variables such as immune cell state, anatomical location, and disease state that govern differences in migration patterns. In all cases, a major factor is the expression of cell surface receptors and their cognate ligands. Rapid adaptation to environmental conditions partly depends on intrinsic cellular immune factors that affect a cell's ability to adjust to new environment. In this review, we discuss both myeloid and lymphoid cells and outline key determinants that govern immune cell migration, including molecules required for immune cell adhesion, modes of migration, chemotaxis, and specific chemokine signaling. Furthermore, we summarize tumor-specific elements that contribute to immune cell trafficking to cancer, while also exploring microenvironment factors that can alter these cellular dynamics within the tumor in both a pro and antitumor fashion. Specifically, we highlight the importance of the secretome in these later aspects. This review considers a myriad of factors that impact immune cell trajectory in cancer. We aim to highlight the immunotherapeutic targets that can be harnessed to achieve controlled immune trafficking to and within tumors.
Keywords: MDSC; T cell; cancer; chemokine; chemotaxis; immune cell; leukocyte; migration; myeloid-derived suppressor cell; trafficking; tumor; tumor microenvironment.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
The Role of CXC Chemokine Receptors 1-4 on Immune Cells in the Tumor Microenvironment.Front Immunol. 2018 Sep 25;9:2159. doi: 10.3389/fimmu.2018.02159. eCollection 2018. Front Immunol. 2018. PMID: 30319622 Free PMC article. Review.
-
Emerging role of mTOR in tumor immune contexture: Impact on chemokine-related immune cells migration.Theranostics. 2020 May 15;10(14):6231-6244. doi: 10.7150/thno.45219. eCollection 2020. Theranostics. 2020. PMID: 32483450 Free PMC article. Review.
-
Dendritic Cells in the Tumor Microenvironment.Adv Exp Med Biol. 2020;1273:29-38. doi: 10.1007/978-3-030-49270-0_2. Adv Exp Med Biol. 2020. PMID: 33119874
-
Chemokines in cancer.Cancer Immunol Res. 2014 Dec;2(12):1125-31. doi: 10.1158/2326-6066.CIR-14-0160. Cancer Immunol Res. 2014. PMID: 25480554 Free PMC article. Review.
-
Myeloid regulatory cells in tumor spreading and metastasis.Immunobiology. 2015 Feb;220(2):236-42. doi: 10.1016/j.imbio.2014.07.017. Epub 2014 Jul 23. Immunobiology. 2015. PMID: 25178934 Review.
Cited by
-
Immune dynamics shaping pre-metastatic and metastatic niches in liver metastases: from molecular mechanisms to therapeutic strategies.Mol Cancer. 2024 Nov 14;23(1):254. doi: 10.1186/s12943-024-02171-z. Mol Cancer. 2024. PMID: 39543660 Free PMC article. Review.
-
The Laws of Attraction: Chemokines as Critical Mediators in Cancer Progression and Immunotherapy Response in Bladder Cancer.Cancers (Basel). 2024 Sep 27;16(19):3303. doi: 10.3390/cancers16193303. Cancers (Basel). 2024. PMID: 39409924 Free PMC article. Review.
-
Single-cell transcriptomics analysis reveals that the tumor-infiltrating B cells determine the indolent fate of papillary thyroid carcinoma.J Exp Clin Cancer Res. 2025 Mar 11;44(1):91. doi: 10.1186/s13046-025-03341-7. J Exp Clin Cancer Res. 2025. PMID: 40069827 Free PMC article.
-
Recent Treatment Strategies and Molecular Pathways in Resistance Mechanisms of Antiangiogenic Therapies in Glioblastoma.Cancers (Basel). 2024 Aug 27;16(17):2975. doi: 10.3390/cancers16172975. Cancers (Basel). 2024. PMID: 39272834 Free PMC article. Review.
-
Migration Dynamics of Human NK Cell Preparations in Microchannels and Their Invasion Into Patient-Derived Tissue.J Cell Mol Med. 2025 Apr;29(7):e70481. doi: 10.1111/jcmm.70481. J Cell Mol Med. 2025. PMID: 40159644 Free PMC article.
References
-
- Simula L., Fumagalli M., Vimeux L., Rajnpreht I., Icard P., Birsen G., An D., Pendino F., Rouault A., Bercovici N., et al. Mitochondrial metabolism sustains CD8(+) T cell migration for an efficient infiltration into solid tumors. Nat. Commun. 2024;15:2203. doi: 10.1038/s41467-024-46377-7. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

