Evaluation of Human Platelet Lysate as an Alternative to Fetal Bovine Serum for Potential Clinical Applications of Stem Cells from Human Exfoliated Deciduous Teeth
- PMID: 38786069
- PMCID: PMC11120611
- DOI: 10.3390/cells13100847
Evaluation of Human Platelet Lysate as an Alternative to Fetal Bovine Serum for Potential Clinical Applications of Stem Cells from Human Exfoliated Deciduous Teeth
Abstract
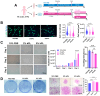
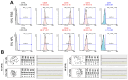
In recent years, there has been a surge in demand for and research focus on cell therapy, driven by the tissue-regenerative and disease-treating potentials of stem cells. Among the candidates, dental pulp stem cells (DPSCs) or human exfoliated deciduous teeth (SHED) have garnered significant attention due to their easy accessibility (non-invasive), multi-lineage differentiation capability (especially neurogenesis), and low immunogenicity. Utilizing these stem cells for clinical purposes requires careful culture techniques such as excluding animal-derived supplements. Human platelet lysate (hPL) has emerged as a safer alternative to fetal bovine serum (FBS) for cell culture. In our study, we assessed the impact of hPL as a growth factor supplement for culture medium, also conducting a characterization of SHED cultured in hPL-supplemented medium (hPL-SHED). The results showed that hPL has effects in enhancing cell proliferation and migration and increasing cell survivability in oxidative stress conditions induced by H2O2. The morphology of hPL-SHED exhibited reduced size and elongation, with a differentiation capacity comparable to or even exceeding that of SHED cultured in a medium supplemented with fetal bovine serum (FBS-SHED). Moreover, no evidence of chromosome abnormalities or tumor formation was detected. In conclusion, hPL-SHED emerges as a promising candidate for cell therapy, exhibiting considerable potential for clinical investigation.
Keywords: DPSC; FBS alternative; SHED; cell therapy; clinical application of stem cells; hPL; human dental pulp stem cells; human platelet lysate; stem cells derived from human exfoliated deciduous teeth.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

