Current and Future Therapeutics for Treating Patients with Sickle Cell Disease
- PMID: 38786070
- PMCID: PMC11120250
- DOI: 10.3390/cells13100848
Current and Future Therapeutics for Treating Patients with Sickle Cell Disease
Abstract
Sickle cell disease (SCD) is the most common genetic blood disorder in the United States, with over 100,000 people suffering from this debilitating disease. SCD is caused by abnormal hemoglobin (Hb) variants that interfere with normal red blood cell (RBC) function. Research on SCD has led to the development and approval of several new SCD therapies in recent years. The recent FDA-approved novel gene therapies are potentially curative, giving patients an additional option besides a hematopoietic bone marrow transplant. Despite the promise of existing therapies, questions remain regarding their long-term pharmacological effects on adults and children. These questions, along with the exorbitant cost of the new gene therapies, justify additional research into more effective therapeutic options. Continual research in this field focuses on not only developing cheaper, more effective cures/treatments but also investigating the physiological effects of the current therapies on SCD patients, particularly on the brain and kidneys. In this article, we undertake a comprehensive review of ongoing clinical trials with completion dates in 2024 or later. Our exploration provides insights into the landscape of current therapeutics and emerging novel therapies designed to combat and potentially eradicate SCD, including the latest FDA-approved gene therapies.
Keywords: clinical trials; gene therapy; hydroxyurea; sickle cell disease; therapeutics; vaso-occlusion; voxelotor.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

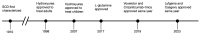

References
-
- Ashorobi D., Ramsey A., Yarrarapu S.N.S., Bhatt R. StatPearls [Internet] StatPearls Publishing; Treasure Island, FL, USA: 2024. Sickle Cell Trait. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

