Antibody-Drug Conjugate Made of Zoledronic Acid and the Anti-CD30 Brentuximab-Vedotin Exert Anti-Lymphoma and Immunostimulating Effects
- PMID: 38786084
- PMCID: PMC11119185
- DOI: 10.3390/cells13100862
Antibody-Drug Conjugate Made of Zoledronic Acid and the Anti-CD30 Brentuximab-Vedotin Exert Anti-Lymphoma and Immunostimulating Effects
Abstract
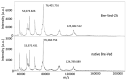
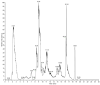
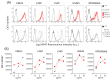
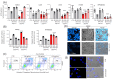
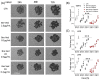
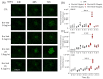
Relevant advances have been made in the management of relapsed/refractory (r/r) Hodgkin Lymphomas (HL) with the use of the anti-CD30 antibody-drug conjugate (ADC) brentuximab-vedotin (Bre-Ved). Unfortunately, most patients eventually progress despite the excellent response rates and tolerability. In this report, we describe an ADC composed of the aminobisphosphonate zoledronic acid (ZA) conjugated to Bre-Ved by binding the free amino groups of this antibody with the phosphoric group of ZA. Liquid chromatography-mass spectrometry, inductively coupled plasma-mass spectrometry, and matrix-assisted laser desorption ionization-mass spectrometry analyses confirmed the covalent linkage between the antibody and ZA. The novel ADC has been tested for its reactivity with the HL/CD30+ lymphoblastoid cell lines (KMH2, L428, L540, HS445, and RPMI6666), showing a better titration than native Bre-Ved. Once the HL-cells are entered, the ADC co-localizes with the lysosomal LAMP1 in the intracellular vesicles. Also, this ADC exerted a stronger anti-proliferative and pro-apoptotic (about one log fold) effect on HL-cell proliferation compared to the native antibody Bre-Ved. Eventually, Bre-Ved-ZA ADC, in contrast with the native antibody, can trigger the proliferation and activation of cytolytic activity of effector-memory Vδ2 T-lymphocytes against HL-cell lines. These findings may support the potential use of this ADC in the management of r/r HL.
Keywords: ADC; CD30; Hodgkin lymphoma(s); Vδ2 T-cells; brentuximab; zoledronic acid.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Loss of or decrease in CD30 expression in four patients with anaplastic large cell lymphoma after brentuximab vedotin-containing therapy.Virchows Arch. 2024 Mar;484(3):465-473. doi: 10.1007/s00428-024-03764-1. Epub 2024 Feb 13. Virchows Arch. 2024. PMID: 38349387
-
Challenges of CD30 expression and its impact on targeted treatment responses in non-Hodgkin lymphoma: New perspectives for evaluation and validation.Pathol Res Pract. 2025 Aug;272:156098. doi: 10.1016/j.prp.2025.156098. Epub 2025 Jun 29. Pathol Res Pract. 2025. PMID: 40618427 Review.
-
Experience of brentuximab vedotin in relapsed/refractory Hodgkin lymphoma and relapsed/refractory systemic anaplastic large-cell lymphoma in the Named Patient Program: Review of the literature.Crit Rev Oncol Hematol. 2015 Sep;95(3):359-69. doi: 10.1016/j.critrevonc.2015.03.011. Epub 2015 Apr 27. Crit Rev Oncol Hematol. 2015. PMID: 25964164
-
Nivolumab for adults with Hodgkin's lymphoma (a rapid review using the software RobotReviewer).Cochrane Database Syst Rev. 2018 Jul 12;7(7):CD012556. doi: 10.1002/14651858.CD012556.pub2. Cochrane Database Syst Rev. 2018. PMID: 30001476 Free PMC article.
-
Brentuximab vedotin for treatment of non-Hodgkin lymphomas: A systematic review.Crit Rev Oncol Hematol. 2017 Jan;109:42-50. doi: 10.1016/j.critrevonc.2016.11.009. Epub 2016 Nov 21. Crit Rev Oncol Hematol. 2017. PMID: 28010897 Free PMC article.
Cited by
-
Liquid biopsy - a narrative review with an update on current US governmental clinical trials targeting immunotherapy.Future Sci OA. 2025 Dec;11(1):2527598. doi: 10.1080/20565623.2025.2527598. Epub 2025 Aug 7. Future Sci OA. 2025. PMID: 40772765 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

