Cytosolic and Acrosomal pH Regulation in Mammalian Sperm
- PMID: 38786087
- PMCID: PMC11120249
- DOI: 10.3390/cells13100865
Cytosolic and Acrosomal pH Regulation in Mammalian Sperm
Abstract
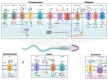
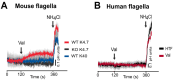
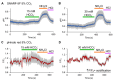
As in most cells, intracellular pH regulation is fundamental for sperm physiology. Key sperm functions like swimming, maturation, and a unique exocytotic process, the acrosome reaction, necessary for gamete fusion, are deeply influenced by pH. Sperm pH regulation, both intracellularly and within organelles such as the acrosome, requires a coordinated interplay of various transporters and channels, ensuring that this cell is primed for fertilization. Consistent with the pivotal importance of pH regulation in mammalian sperm physiology, several of its unique transporters are dependent on cytosolic pH. Examples include the Ca2+ channel CatSper and the K+ channel Slo3. The absence of these channels leads to male infertility. This review outlines the main transport elements involved in pH regulation, including cytosolic and acrosomal pH, that participate in these complex functions. We present a glimpse of how these transporters are regulated and how distinct sets of them are orchestrated to allow sperm to fertilize the egg. Much research is needed to begin to envision the complete set of players and the choreography of how cytosolic and organellar pH are regulated in each sperm function.
Keywords: acrosomal pH; bicarbonate transport; cytosolic pH; mammalian sperm capacitation; proton channels and transporters.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Two-pore channel 1 and Ca2+ release-activated Ca2+ channels contribute to the acrosomal pH-dependent intracellular Ca2+ increase in mouse sperm.J Physiol. 2023 Jul;601(14):2935-2958. doi: 10.1113/JP284247. Epub 2023 Jun 6. J Physiol. 2023. PMID: 37278367
-
Intracellular Ca(2+)-Mg(2+)-ATPase regulates calcium influx and acrosomal exocytosis in bull and ram spermatozoa.Biol Reprod. 1999 Nov;61(5):1226-34. doi: 10.1095/biolreprod61.5.1226. Biol Reprod. 1999. PMID: 10529268
-
Acrosomal alkalinization occurs during human sperm capacitation.Mol Hum Reprod. 2022 Mar 8;28(3):gaac005. doi: 10.1093/molehr/gaac005. Mol Hum Reprod. 2022. PMID: 35201340
-
pH Homeodynamics and Male Fertility: A Coordinated Regulation of Acid-Based Balance during Sperm Journey to Fertilization.Biomolecules. 2024 Jun 12;14(6):685. doi: 10.3390/biom14060685. Biomolecules. 2024. PMID: 38927088 Free PMC article. Review.
-
Intracellular pH in sperm physiology.Biochem Biophys Res Commun. 2014 Aug 1;450(3):1149-58. doi: 10.1016/j.bbrc.2014.05.100. Epub 2014 Jun 2. Biochem Biophys Res Commun. 2014. PMID: 24887564 Free PMC article. Review.
Cited by
-
Decoding a novel non-enzymatic protein acetylation mechanism in sperm that is essential for fertilizing potential.Biol Res. 2025 May 29;58(1):30. doi: 10.1186/s40659-025-00613-6. Biol Res. 2025. PMID: 40442844 Free PMC article.
-
Activation of motility and chemotaxis in the spermatozoa.Reprod Med Biol. 2025 Mar 5;24(1):e12638. doi: 10.1002/rmb2.12638. eCollection 2025 Jan-Dec. Reprod Med Biol. 2025. PMID: 40045950 Free PMC article. Review.
-
Citrate Promotes Nitric Oxide Production during Human Sperm Capacitation.Antioxidants (Basel). 2024 Jul 23;13(8):885. doi: 10.3390/antiox13080885. Antioxidants (Basel). 2024. PMID: 39199131 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
- IN207122/Dirección General de Asuntos del Personal Académico (DGAPA) de la Universidad Nacional Autónoma de México (UNAM)-Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica (PAPIIT)
- HD038082-17A1/NH/NIH HHS/United States
- CF-2023-I-291/Consejo Nacional de Humanidades, Ciencias y Tecnologías
- R01 HD038082/HD/NICHD NIH HHS/United States
- IN218123/Dirección General de Asuntos del Personal Académico (DGAPA) de la Universidad Nacional Autónoma de México (UNAM)-Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica (PAPIIT)
- IN205722/Dirección General de Asuntos del Personal Académico (DGAPA) de la Universidad Nacional Autónoma de México (UNAM)-Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica (PAPIIT)
- PE206424/Dirección General de Asuntos del Personal Académico (DGAPA) de la Universidad Nacional Autónoma de México (UNAM)-Programa de Apoyo a Proyectos para Innovar y Mejorar la Educación (PAPIME)
- IN204922/Dirección General de Asuntos del Personal Académico (DGAPA) de la Universidad Nacional Autónoma de México (UNAM)-Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica (PAPIIT)
- A1-S-8768/Consejo Nacional de Humanidades, Ciencias y Tecnologías
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

