Deciphering the Molecular Nexus: An In-Depth Review of Mitochondrial Pathways and Their Role in Cell Death Crosstalk
- PMID: 38786088
- PMCID: PMC11119937
- DOI: 10.3390/cells13100863
Deciphering the Molecular Nexus: An In-Depth Review of Mitochondrial Pathways and Their Role in Cell Death Crosstalk
Abstract
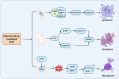
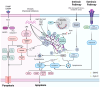
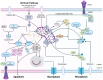
Cellular demise is a pivotal event in both developmental processes and disease states, with mitochondrial regulation playing an essential role. Traditionally, cell death was categorized into distinct types, considered to be linear and mutually exclusive pathways. However, the current understanding has evolved to recognize the complex and interconnected mechanisms of cell death, especially within apoptosis, pyroptosis, and necroptosis. Apoptosis, pyroptosis, and necroptosis are governed by intricate molecular pathways, with mitochondria acting as central decision-makers in steering cells towards either apoptosis or pyroptosis through various mediators. The choice between apoptosis and necroptosis is often determined by mitochondrial signaling and is orchestrated by specific proteins. The molecular dialogue and the regulatory influence of mitochondria within these cell death pathways are critical research areas. Comprehending the shared elements and the interplay between these death modalities is crucial for unraveling the complexities of cellular demise.
Keywords: apoptosis; mitochondria; necroptosis; pyroptosis.
Conflict of interest statement
All authors declare that there are no competing interests associated with the manuscript.
Figures
Similar articles
-
Navigating the complexities of cell death: Insights into accidental and programmed cell death.Tissue Cell. 2024 Dec;91:102586. doi: 10.1016/j.tice.2024.102586. Epub 2024 Oct 16. Tissue Cell. 2024. PMID: 39426124 Review.
-
Mitochondria and cell death.Nat Cell Biol. 2024 Sep;26(9):1434-1446. doi: 10.1038/s41556-024-01429-4. Epub 2024 Jun 20. Nat Cell Biol. 2024. PMID: 38902422 Review.
-
Apoptosis, Pyroptosis, and Necroptosis-Oh My! The Many Ways a Cell Can Die.J Mol Biol. 2022 Feb 28;434(4):167378. doi: 10.1016/j.jmb.2021.167378. Epub 2021 Nov 25. J Mol Biol. 2022. PMID: 34838807 Review.
-
Necroptosis, pyroptosis and apoptosis: an intricate game of cell death.Cell Mol Immunol. 2021 May;18(5):1106-1121. doi: 10.1038/s41423-020-00630-3. Epub 2021 Mar 30. Cell Mol Immunol. 2021. PMID: 33785842 Free PMC article. Review.
-
Pore formation in regulated cell death.EMBO J. 2020 Dec 1;39(23):e105753. doi: 10.15252/embj.2020105753. Epub 2020 Oct 30. EMBO J. 2020. PMID: 33124082 Free PMC article. Review.
Cited by
-
Targeting Mitochondrial Dysfunction in Cerebral Ischemia: Advances in Pharmacological Interventions.Antioxidants (Basel). 2025 Jan 18;14(1):108. doi: 10.3390/antiox14010108. Antioxidants (Basel). 2025. PMID: 39857442 Free PMC article. Review.
-
The interplay between α-synuclein aggregation and necroptosis in Parkinson's disease: a spatiotemporal perspective.Front Neurosci. 2025 Apr 8;19:1567445. doi: 10.3389/fnins.2025.1567445. eCollection 2025. Front Neurosci. 2025. PMID: 40264913 Free PMC article. Review.
-
Targeting Cancer Cell Fate: Apoptosis, Autophagy, and Gold Nanoparticles in Treatment Strategies.Curr Issues Mol Biol. 2025 Jun 14;47(6):460. doi: 10.3390/cimb47060460. Curr Issues Mol Biol. 2025. PMID: 40699859 Free PMC article. Review.
References
-
- Galluzzi L., Vitale I., Aaronson S.A., Abrams J.M., Adam D., Agostinis P., Alnemri E.S., Altucci L., Amelio I., Andrews D.W., et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25:486–541. doi: 10.1038/s41418-017-0012-4. - DOI - PMC - PubMed
-
- Koren E., Fuchs Y. Modes of Regulated Cell Death in Cancer. Cancer Discov. 2021;11:245–265. doi: 10.1158/2159-8290.CD-20-0789. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

