Retinoid Synthesis Regulation by Retinal Cells in Health and Disease
- PMID: 38786093
- PMCID: PMC11120330
- DOI: 10.3390/cells13100871
Retinoid Synthesis Regulation by Retinal Cells in Health and Disease
Abstract
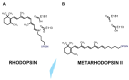
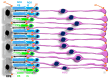
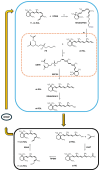
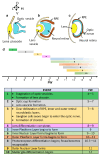
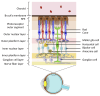
Vision starts in retinal photoreceptors when specialized proteins (opsins) sense photons via their covalently bonded vitamin A derivative 11cis retinaldehyde (11cis-RAL). The reaction of non-enzymatic aldehydes with amino groups lacks specificity, and the reaction products may trigger cell damage. However, the reduced synthesis of 11cis-RAL results in photoreceptor demise and suggests the need for careful control over 11cis-RAL handling by retinal cells. This perspective focuses on retinoid(s) synthesis, their control in the adult retina, and their role during retina development. It also explores the potential importance of 9cis vitamin A derivatives in regulating retinoid synthesis and their impact on photoreceptor development and survival. Additionally, recent advancements suggesting the pivotal nature of retinoid synthesis regulation for cone cell viability are discussed.
Keywords: 11cis retinaldehyde; 9cis retinaldehyde; Müller glial cells; RPE; RPE65; cone photoreceptors; retinal dystrophies; retinoic acid; rod photoreceptors; vision.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Peropsin modulates transit of vitamin A from retina to retinal pigment epithelium.J Biol Chem. 2017 Dec 29;292(52):21407-21416. doi: 10.1074/jbc.M117.812701. Epub 2017 Nov 6. J Biol Chem. 2017. PMID: 29109151 Free PMC article.
-
Prolonged Inner Retinal Photoreception Depends on the Visual Retinoid Cycle.J Neurosci. 2016 Apr 13;36(15):4209-17. doi: 10.1523/JNEUROSCI.2629-14.2016. J Neurosci. 2016. PMID: 27076420 Free PMC article.
-
Cell-type- and developmental-stage-specific metabolism and storage of retinoids by embryonic chick retinal cells in culture.Exp Eye Res. 1994 Jun;58(6):675-87. doi: 10.1006/exer.1994.1065. Exp Eye Res. 1994. PMID: 7925707
-
Vitamin A metabolism in rod and cone visual cycles.Annu Rev Nutr. 2012 Aug 21;32:125-45. doi: 10.1146/annurev-nutr-071811-150748. Annu Rev Nutr. 2012. PMID: 22809103 Review.
-
Retinal photodamage mediated by all-trans-retinal.Photochem Photobiol. 2012 Nov-Dec;88(6):1309-19. doi: 10.1111/j.1751-1097.2012.01143.x. Epub 2012 Apr 24. Photochem Photobiol. 2012. PMID: 22428905 Free PMC article. Review.
Cited by
-
Biosynthesis of fatty aldehydes and alcohols in the eye and their role in meibogenesis.J Biol Chem. 2025 Jul;301(7):110330. doi: 10.1016/j.jbc.2025.110330. Epub 2025 Jun 3. J Biol Chem. 2025. PMID: 40473210 Free PMC article.
-
Introducing a Porcine Inflammatory Ex Vivo Retina Model for Diabetic Retinopathy.Int J Mol Sci. 2025 Apr 21;26(8):3919. doi: 10.3390/ijms26083919. Int J Mol Sci. 2025. PMID: 40332752 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

