Heterologous HSPC Transplantation Rescues Neuroinflammation and Ameliorates Peripheral Manifestations in the Mouse Model of Lysosomal Transmembrane Enzyme Deficiency, MPS IIIC
- PMID: 38786099
- PMCID: PMC11120110
- DOI: 10.3390/cells13100877
Heterologous HSPC Transplantation Rescues Neuroinflammation and Ameliorates Peripheral Manifestations in the Mouse Model of Lysosomal Transmembrane Enzyme Deficiency, MPS IIIC
Abstract
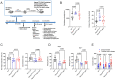
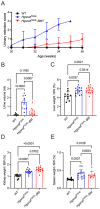
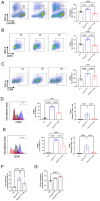
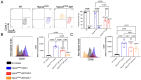
Mucopolysaccharidosis III type C (MPS IIIC) is an untreatable neuropathic lysosomal storage disease caused by a genetic deficiency of the lysosomal N-acetyltransferase, HGSNAT, catalyzing a transmembrane acetylation of heparan sulfate. HGSNAT is a transmembrane enzyme incapable of free diffusion between the cells or their cross-correction, which limits development of therapies based on enzyme replacement and gene correction. Since our previous work identified neuroinflammation as a hallmark of the CNS pathology in MPS IIIC, we tested whether it can be corrected by replacement of activated brain microglia with neuroprotective macrophages/microglia derived from a heterologous HSPC transplant. Eight-week-old MPS IIIC (HgsnatP304L) mice were transplanted with HSPC from congenic wild type mice after myeloablation with Busulfan and studied using behavior test battery, starting from the age of 6 months. At the age of ~8 months, mice were sacrificed to study pathological changes in the brain, heparan sulfate storage, and other biomarkers of the disease. We found that the treatment corrected several behavior deficits including hyperactivity and reduction in socialization, but not memory decline. It also improved several features of CNS pathology such as microastroglyosis, expression of pro-inflammatory cytokine IL-1β, and accumulation of misfolded amyloid aggregates in cortical neurons. At the periphery, the treatment delayed development of terminal urinary retention, potentially increasing longevity, and reduced blood levels of heparan sulfate. However, we did not observe correction of lysosomal storage phenotype in neurons and heparan sulfate brain levels. Together, our results demonstrate that neuroinflammation in a neurological lysosomal storage disease, caused by defects in a transmembrane enzyme, can be effectively ameliorated by replacement of microglia bearing the genetic defect with cells from a normal healthy donor. They also suggest that heterologous HSPC transplant, if used together with other methods, such as chaperone therapy or substrate reduction therapy, may constitute an effective combination therapy for MPS IIIC and other disorders with a similar etiology.
Keywords: HSPC; Sanfilippo disease; allogenic HSPC transplantation; heparan sulfate; microglia; mucopolysaccharidosis; neuroinflammation.
Conflict of interest statement
A.V.P. received honoraria and research contracts from Phoenix Nest Inc involved in development of therapies for MPS IIID and IIIC. Authors have no additional financial interests.
Figures

Similar articles
-
Transplantation of Wild-Type Hematopoietic Stem and Progenitor Cells Improves Disease Phenotypes in a Mucopolysaccharidosis IIIC Mouse Model.Cell Transplant. 2025 Jan-Dec;34:9636897251323966. doi: 10.1177/09636897251323966. Epub 2025 Mar 24. Cell Transplant. 2025. PMID: 40126917 Free PMC article.
-
Progressive neurologic and somatic disease in a novel mouse model of human mucopolysaccharidosis type IIIC.Dis Model Mech. 2016 Sep 1;9(9):999-1013. doi: 10.1242/dmm.025171. Epub 2016 Aug 4. Dis Model Mech. 2016. PMID: 27491071 Free PMC article.
-
Lysosomal storage of heparan sulfate causes mitochondrial defects, altered autophagy, and neuronal death in the mouse model of mucopolysaccharidosis III type C.Autophagy. 2016 Jun 2;12(6):1059-60. doi: 10.1080/15548627.2015.1046671. Epub 2015 May 22. Autophagy. 2016. PMID: 25998837 Free PMC article.
-
Glycosaminoglycans and mucopolysaccharidosis type III.Front Biosci (Landmark Ed). 2016 Jun 1;21(7):1393-409. doi: 10.2741/4463. Front Biosci (Landmark Ed). 2016. PMID: 27100513 Review.
-
Sanfilippo syndrome type C: mutation spectrum in the heparan sulfate acetyl-CoA: alpha-glucosaminide N-acetyltransferase (HGSNAT) gene.Hum Mutat. 2009 Jun;30(6):918-25. doi: 10.1002/humu.20986. Hum Mutat. 2009. PMID: 19479962 Review.
Cited by
-
Harnessing microglia-based cell therapies for the treatment of neurodegenerative diseases.Curr Opin Immunol. 2025 Jun;94:102552. doi: 10.1016/j.coi.2025.102552. Epub 2025 Mar 25. Curr Opin Immunol. 2025. PMID: 40138748 Review.
-
Combined treatment of Ketogenic diet and propagermanium reduces neuroinflammation in Tay-Sachs disease mouse model.Metab Brain Dis. 2025 Feb 28;40(3):133. doi: 10.1007/s11011-025-01553-6. Metab Brain Dis. 2025. PMID: 40019557 Free PMC article.
-
Transplantation of Wild-Type Hematopoietic Stem and Progenitor Cells Improves Disease Phenotypes in a Mucopolysaccharidosis IIIC Mouse Model.Cell Transplant. 2025 Jan-Dec;34:9636897251323966. doi: 10.1177/09636897251323966. Epub 2025 Mar 24. Cell Transplant. 2025. PMID: 40126917 Free PMC article.
References
-
- Ausseil J., Desmaris N., Bigou S., Attali R., Corbineau S., Vitry S., Parent M., Cheillan D., Fuller M., Maire I., et al. Early Neurodegeneration Progresses Independently of Microglial Activation by Heparan Sulfate in the Brain of Mucopolysaccharidosis Iiib Mice. PLoS ONE. 2008;3:e2296. doi: 10.1371/journal.pone.0002296. - DOI - PMC - PubMed
-
- Martins C., Hulkova H., Dridi L., Dormoy-Raclet V., Grigoryeva L., Choi Y., Langford-Smith A., Wilkinson F.L., Ohmi K., DiCristo G., et al. Neuroinflammation, Mitochondrial Defects and Neurodegeneration in Mucopolysaccharidosis Iii Type C Mouse Model. Brain. 2015;138:336–355. doi: 10.1093/brain/awu355. - DOI - PMC - PubMed
-
- Baregamian N., Song J., Bailey C.E., Papaconstantinou J., Evers B.M., Chung D.H. Tumor Necrosis Factor-Alpha and Apoptosis Signal-Regulating Kinase 1 Control Reactive Oxygen Species Release, Mitochondrial Autophagy, and C-Jun N-Terminal Kinase/P38 Phosphorylation During Necrotizing Enterocolitis. Oxid. Med. Cell Longev. 2009;2:297–306. doi: 10.4161/oxim.2.5.9541. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

