Pre-Clinical Assessment of SAR442257, a CD38/CD3xCD28 Trispecific T Cell Engager in Treatment of Relapsed/Refractory Multiple Myeloma
- PMID: 38786100
- PMCID: PMC11120574
- DOI: 10.3390/cells13100879
Pre-Clinical Assessment of SAR442257, a CD38/CD3xCD28 Trispecific T Cell Engager in Treatment of Relapsed/Refractory Multiple Myeloma
Abstract
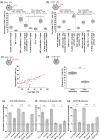
Current treatment strategies for multiple myeloma (MM) are highly effective, but most patients develop relapsed/refractory disease (RRMM). The anti-CD38/CD3xCD28 trispecific antibody SAR442257 targets CD38 and CD28 on MM cells and co-stimulates CD3 and CD28 on T cells (TCs). We evaluated different key aspects such as MM cells and T cells avidity interaction, tumor killing, and biomarkers for drug potency in three distinct cohorts of RRMM patients. We found that a significantly higher proportion of RRMM patients (86%) exhibited aberrant co-expression of CD28 compared to newly diagnosed MM (NDMM) patients (19%). Furthermore, SAR442257 mediated significantly higher TC activation, resulting in enhanced MM killing compared to bispecific functional knockout controls for all relapse cohorts (Pearson's r = 0.7). Finally, patients refractory to anti-CD38 therapy had higher levels of TGF-β (up to 20-fold) compared to other cohorts. This can limit the activity of SAR442257. Vactoserib, a TGF-β inhibitor, was able to mitigate this effect and restore sensitivity to SAR442257 in these experiments. In conclusion, SAR442257 has high potential for enhancing TC cytotoxicity by co-targeting CD38 and CD28 on MM and CD3/CD28 on T cells.
Keywords: T cell engager; cell avidity; microenvironment; refractory multiple myeloma.
Conflict of interest statement
We disclose that Sanofi employees may hold shares and/or stock options in the company. RMR, IS, DS are employed by a commercial company (LUMICKS).
Figures



References
-
- Mateo G., Castellanos M., Rasillo A., Gutierrez N.C., Montalban M.A., Martin M.L., Hernandez J.M., Lopez-Berges M.C., Montejano L., Blade J., et al. Genetic abnormalities and patterns of antigenic expression in multiple myeloma. Clin. Cancer Res. 2005;11:3661–3667. doi: 10.1158/1078-0432.CCR-04-1489. - DOI - PubMed
-
- Almeida J., Orfao A., Ocqueteau M., Mateo G., Corral M., Caballero M.D., Blade J., Moro M.J., Hernandez J., San Miguel J.F. High-sensitive immunophenotyping and DNA ploidy studies for the investigation of minimal residual disease in multiple myeloma. Br. J. Haematol. 1999;107:121–131. doi: 10.1046/j.1365-2141.1999.01685.x. - DOI - PubMed
-
- Chung D.J., Pronschinske K.B., Shyer J.A., Sharma S., Leung S., Curran S.A., Lesokhin A.M., Devlin S.M., Giralt S.A., Young J.W. T-cell Exhaustion in Multiple Myeloma Relapse after Autotransplant: Optimal Timing of Immunotherapy. Cancer Immunol. Res. 2016;4:61–71. doi: 10.1158/2326-6066.CIR-15-0055. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

