NADH Intraperitoneal Injection Prevents Lung Inflammation in a BALB/C Mice Model of Cigarette Smoke-Induced Chronic Obstructive Pulmonary Disease
- PMID: 38786103
- PMCID: PMC11120028
- DOI: 10.3390/cells13100881
NADH Intraperitoneal Injection Prevents Lung Inflammation in a BALB/C Mice Model of Cigarette Smoke-Induced Chronic Obstructive Pulmonary Disease
Abstract
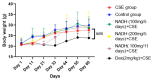
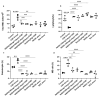
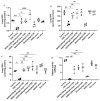
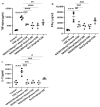
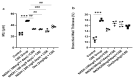
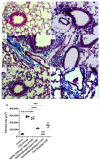
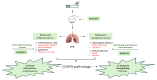
Cigarette smoke is one of the main factors in Chronic Obstructive Pulmonary Disease (COPD), a respiratory syndrome marked by persistent respiratory symptoms and increasing airway obstruction. Perturbed NAD+/NADH levels may play a role in various diseases, including lung disorders like COPD. In our study, we investigated the preventive effect of NADH supplementation in an experimental model of COPD induced by cigarette smoke extract (CSE). N = 64 mice randomly distributed in eight groups were injected with NADH (two doses of 100 mg/kg or 200 mg/kg) or dexamethasone (2 mg/kg) before being exposed to CSE for up to 9 weeks. Additionally, NADH supplementation preserved lung antioxidant defenses by preventing the functional loss of key enzymes such as superoxide dismutase (SOD), glutathione peroxidase (GPX), catalase, and the expression levels of glutathione (GSH) (n = 4, p < 0.001). It also reduced oxidative damage markers, such as malondialdehyde (MDA) and nitrites (n = 4, p < 0.001). A marked increase in tissue myeloperoxidase activity was assessed (MPO), confirming neutrophils implication in the inflammatory process. The latter was significantly ameliorated in the NADH-treated groups (p < 0.001). Finally, NADH prevented the CSE-induced secretion of cytokines such as Tumor Necrosis Factor alpha (TNF-α), IL-17, and IFN-y (n = 4, p < 0.001). Our study shows, for the first time, the clinical potential of NADH supplementation in preventing key features of COPD via its unique anti-inflammatory and antioxidant properties.
Keywords: NADH; animal model; cigarette smoke; inflammation; oxidative stress.
Conflict of interest statement
All authors (except for Birkmayer) declare no competing interests. The NADH used in the present study was a gift from the late Birkmayer, the founder of the Birkmayer NADH Company, who tragically died on 28 August 2022. The Birkmayer NADH Company was not involved in the study conception and design, data collection, analysis and interpretation of results, or manuscript preparation. None of the authors received any financial or project funding support from the Birkmayer NADH Company.
Figures


Similar articles
-
Ergosterol attenuates cigarette smoke extract-induced COPD by modulating inflammation, oxidative stress and apoptosis in vitro and in vivo.Clin Sci (Lond). 2019 Jul 15;133(13):1523-1536. doi: 10.1042/CS20190331. Print 2019 Jul 15. Clin Sci (Lond). 2019. PMID: 31270147
-
Protective effects of liquiritin apioside on cigarette smoke-induced lung epithelial cell injury.Fundam Clin Pharmacol. 2012 Aug;26(4):473-83. doi: 10.1111/j.1472-8206.2011.00956.x. Epub 2011 Jun 1. Fundam Clin Pharmacol. 2012. PMID: 21631586
-
Anemoside B4 prevents chronic obstructive pulmonary disease through alleviating cigarette smoke-induced inflammatory response and airway epithelial hyperplasia.Phytomedicine. 2022 Dec;107:154431. doi: 10.1016/j.phymed.2022.154431. Epub 2022 Sep 2. Phytomedicine. 2022. PMID: 36115169
-
Oxidative Stress in Ozone-Induced Chronic Lung Inflammation and Emphysema: A Facet of Chronic Obstructive Pulmonary Disease.Front Immunol. 2020 Sep 2;11:1957. doi: 10.3389/fimmu.2020.01957. eCollection 2020. Front Immunol. 2020. PMID: 32983127 Free PMC article. Review.
-
Antioxidant therapeutic advances in COPD.Ther Adv Respir Dis. 2008 Dec;2(6):351-74. doi: 10.1177/1753465808098224. Ther Adv Respir Dis. 2008. PMID: 19124382 Free PMC article. Review.
Cited by
-
Inflammation: The Cause of All Diseases.Cells. 2024 Nov 18;13(22):1906. doi: 10.3390/cells13221906. Cells. 2024. PMID: 39594654 Free PMC article.
References
-
- Sidhaye V.K., Koval M. Lung Epithelial Biology in the Pathogenesis of Pulmonary Disease. Academic Press; Cambridge, MA, USA: 2017.
-
- Strzelak A., Ratajczak A., Adamiec A., Feleszko W. Tobacco Smoke Induces and Alters Immune Responses in the Lung Triggering Inflammation, Allergy, Asthma and Other Lung Diseases: A Mechanistic Review. Int. J. Environ. Res. Public Health. 2018;15:1033. doi: 10.3390/ijerph15051033. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

