A Response Surface Methodological Approach for Large-Scale Production of Antibacterials from Lactiplantibacillus plantarum with Potential Utility against Foodborne and Orthopedic Infections
- PMID: 38786166
- PMCID: PMC11118495
- DOI: 10.3390/antibiotics13050437
A Response Surface Methodological Approach for Large-Scale Production of Antibacterials from Lactiplantibacillus plantarum with Potential Utility against Foodborne and Orthopedic Infections
Abstract
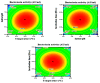
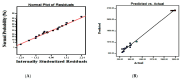
A variety of bacteria, including beneficial probiotic lactobacilli, produce antibacterials to kill competing bacteria. Lactobacilli secrete antimicrobial peptides (AMPs) called bacteriocins and organic acids. In the food industry, bacteriocins, but even whole cell-free supernatants, are becoming more and more important as bio-preservatives, while, in orthopedics, bacteriocins are introducing new perspectives in biomaterials technologies for anti-infective surfaces. Studies are focusing on Lactiplantibacillus plantarum (previously known as Lactobacillus plantarum). L. plantarum exhibits great phenotypic versatility, which enhances the chances for its industrial exploitation. Importantly, more than other lactobacilli, it relies on AMPs for its antibacterial activity. In this study, Response Surface Methodology (RSM) through a Box-Behnken experimental design was used to estimate the optimal conditions for the production of antibacterials by L. plantarum. A temperature of 35 °C, pH 6.5, and an incubation time of 48 h provided the highest concentration of antibacterials. The initial pH was the main factor influencing the production of antibacterials, at 95% confidence level. Thanks to RSM, the titer of antibacterials increased more than 10-fold, this result being markedly higher than those obtained in the very few studies that have so far used similar statistical methodologies. The Box-Behnken design turned out to be a valid model to satisfactorily plan a large-scale production of antibacterials from L. plantarum.
Keywords: Box–Behnken design; Lactiplantibacillus plantarum; antibacterial activity; bacteriocins; foodborne infections; orthopedic implant infections.
Conflict of interest statement
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


References
LinkOut - more resources
Full Text Sources

