Systematic Review and Meta-Analysis Provide no Guidance on Management of Asymptomatic Bacteriuria within the First Year after Kidney Transplantation
- PMID: 38786170
- PMCID: PMC11117648
- DOI: 10.3390/antibiotics13050442
Systematic Review and Meta-Analysis Provide no Guidance on Management of Asymptomatic Bacteriuria within the First Year after Kidney Transplantation
Abstract
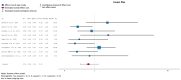
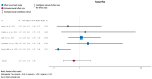
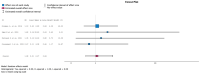
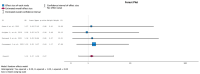
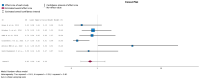
(1) Background: Urinary tract infections (UTIs) are among the most frequent complications in kidney transplant (KT) recipients. Asymptomatic bacteriuria (ASB) may be a risk factor for UTIs and graft rejection. We aimed to evaluate available evidence regarding the benefit of screening and treatment of ASB within the first year after KT. (2) Evidence acquisition: A systematic literature search was conducted in MEDLINE, the Cochrane Library CENTRAL and Embase. Inclusion criteria were manuscripts in English addressing the management of ASB after KT. The PICO questions concerned Patients (adults receiving a KT), Intervention (screening, diagnosis and treatment of ASB), Control (screening and no antibiotic treatment) and Outcome (UTIs, sepsis, kidney failure and death). (3) Evidence synthesis: The systematic review identified 151 studies, and 16 full-text articles were evaluated. Seven were excluded because they did not evaluate the effect of treatment of ASB. There was no evidence for a higher incidence of lower UTIs, acute pyelonephritis, graft loss, or mortality in patients not treated with antibiotics for ASB. Analysis of comparative non-randomized and observational studies did not provide supplementary evidence to guide clinical recommendations. We believe this lack of evidence is due to confounding risk factors that are not being considered in the stratification of study patients.
Keywords: antibiotics; asymptomatic bacteriuria (ASB); graft rejection; kidney failure; kidney transplant; meta-analysis; systematic review; urinary tract infection.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Gan V.H.L., Shoskes D.A. Urogenital Infections and Inflammations GMS. 2018. [(accessed on 23 April 2024)]. UTI in renal transplant patients. Available online: https://books.publisso.de/publisso_gold/book/52/chapter/12.
Publication types
LinkOut - more resources
Full Text Sources

