Reducing Inert Materials for Optimal Cell-Cell and Cell-Matrix Interactions within Microphysiological Systems
- PMID: 38786472
- PMCID: PMC11118140
- DOI: 10.3390/biomimetics9050262
Reducing Inert Materials for Optimal Cell-Cell and Cell-Matrix Interactions within Microphysiological Systems
Abstract
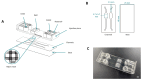
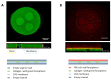
In the pursuit of achieving a more realistic in vitro simulation of human biological tissues, microfluidics has emerged as a promising technology. Organ-on-a-chip (OoC) devices, a product of this technology, contain miniature tissues within microfluidic chips, aiming to closely mimic the in vivo environment. However, a notable drawback is the presence of inert material between compartments, hindering complete contact between biological tissues. Current membranes, often made of PDMS or plastic materials, prevent full interaction between cell types and nutrients. Furthermore, their non-physiological mechanical properties and composition may induce unexpected cell responses. Therefore, it is essential to minimize the contact area between cells and the inert materials while simultaneously maximizing the direct contact between cells and matrices in different compartments. The main objective of this work is to minimize inert materials within the microfluidic chip while preserving proper cellular distribution. Two microfluidic devices were designed, each with a specific focus on maximizing direct cell-matrix or cell-cell interactions. The first chip, designed to increase direct cell-cell interactions, incorporates a nylon mesh with regular pores of 150 microns. The second chip minimizes interference from inert materials, thereby aiming to increase direct cell-matrix contact. It features an inert membrane with optimized macropores of 1 mm of diameter for collagen hydrogel deposition. Biological validation of both devices has been conducted through the implementation of cell migration and cell-to-cell interaction assays, as well as the development of epithelia, from isolated cells or spheroids. This endeavor contributes to the advancement of microfluidic technology, aimed at enhancing the precision and biological relevance of in vitro simulations in pursuit of more biomimetic models.
Keywords: inert material; macro/micropores; membranes; mesh; microfluidic devices; microphysiological systems (MPS); migration; organ-on-a-chip (OoC); spheroids.
Conflict of interest statement
I. Ochoa, R. Monge and S. Oliván are promoters and consultants for BEOnChip S.L.
Figures







Similar articles
-
Modular Microphysiological System for Modeling of Biologic Barrier Function.Front Bioeng Biotechnol. 2020 Nov 12;8:581163. doi: 10.3389/fbioe.2020.581163. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 33304889 Free PMC article.
-
Integration of Electrospun Membranes into Low-Absorption Thermoplastic Organ-on-Chip.ACS Biomater Sci Eng. 2021 Jul 12;7(7):3006-3017. doi: 10.1021/acsbiomaterials.0c01062. Epub 2021 Feb 16. ACS Biomater Sci Eng. 2021. PMID: 33591723
-
Erratum: Scalable Fabrication of Stretchable, Dual Channel, Microfluidic Organ Chips.J Vis Exp. 2019 May 8;(147). doi: 10.3791/6296. J Vis Exp. 2019. PMID: 31067212
-
Applications of Polymers for Organ-on-Chip Technology in Urology.Polymers (Basel). 2022 Apr 20;14(9):1668. doi: 10.3390/polym14091668. Polymers (Basel). 2022. PMID: 35566836 Free PMC article. Review.
-
Engineering Tissue Barrier Models on Hydrogel Microfluidic Platforms.ACS Appl Mater Interfaces. 2021 Mar 31;13(12):13920-13933. doi: 10.1021/acsami.0c21573. Epub 2021 Mar 19. ACS Appl Mater Interfaces. 2021. PMID: 33739812 Review.
References
Grants and funding
- Proyect PID2021-126051OB-C41 funded by MCIN /AEI /10.Proyect PID2021-126051OB-C41 funded by MCIN /AEI /10.13039/501100011033 / FEDER, UE13039/501100011033 / FEDER, UE/Ministry of Science and Innovation, the European Regional Development Fund
- No 87619 (M4M: MOORE4MEDICAL H2020-EU.2.1.1.7. - ECSEL/European Union's Horizon 2020
- 778354 (CISTEM H2020-MSCA-RISE-201/European Union's Horizon 2020
LinkOut - more resources
Full Text Sources

