Advancement in Cancer Vasculogenesis Modeling through 3D Bioprinting Technology
- PMID: 38786516
- PMCID: PMC11118135
- DOI: 10.3390/biomimetics9050306
Advancement in Cancer Vasculogenesis Modeling through 3D Bioprinting Technology
Abstract
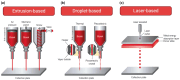
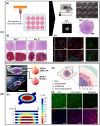
Cancer vasculogenesis is a pivotal focus of cancer research and treatment given its critical role in tumor development, metastasis, and the formation of vasculogenic microenvironments. Traditional approaches to investigating cancer vasculogenesis face significant challenges in accurately modeling intricate microenvironments. Recent advancements in three-dimensional (3D) bioprinting technology present promising solutions to these challenges. This review provides an overview of cancer vasculogenesis and underscores the importance of precise modeling. It juxtaposes traditional techniques with 3D bioprinting technologies, elucidating the advantages of the latter in developing cancer vasculogenesis models. Furthermore, it explores applications in pathological investigations, preclinical medication screening for personalized treatment and cancer diagnostics, and envisages future prospects for 3D bioprinted cancer vasculogenesis models. Despite notable advancements, current 3D bioprinting techniques for cancer vasculogenesis modeling have several limitations. Nonetheless, by overcoming these challenges and with technological advances, 3D bioprinting exhibits immense potential for revolutionizing the understanding of cancer vasculogenesis and augmenting treatment modalities.
Keywords: 3D bioprinting technology; cancer; cancer microenvironment; cancer modeling; vasculogenesis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
Advancements in Research on Constructing Physiological and Pathological Liver Models and Their Applications Utilizing Bioprinting Technology.Molecules. 2023 Apr 24;28(9):3683. doi: 10.3390/molecules28093683. Molecules. 2023. PMID: 37175094 Free PMC article. Review.
-
Spheroid-Hydrogel-Integrated Biomimetic System: A New Frontier in Advanced Three-Dimensional Cell Culture Technology.Cells Tissues Organs. 2025;214(2):128-147. doi: 10.1159/000541416. Epub 2024 Sep 12. Cells Tissues Organs. 2025. PMID: 39265553 Free PMC article. Review.
-
Advances in tumor microenvironment: Applications and challenges of 3D bioprinting.Biochem Biophys Res Commun. 2024 Oct 20;730:150339. doi: 10.1016/j.bbrc.2024.150339. Epub 2024 Jul 8. Biochem Biophys Res Commun. 2024. PMID: 39032359 Review.
-
Improving Bioprinted Volumetric Tumor Microenvironments In Vitro.Trends Cancer. 2020 Sep;6(9):745-756. doi: 10.1016/j.trecan.2020.06.002. Epub 2020 Jul 14. Trends Cancer. 2020. PMID: 32680649 Free PMC article. Review.
-
Recent advances in 3D bioprinting for cancer research: From precision models to personalized therapies.Drug Discov Today. 2024 Apr;29(4):103924. doi: 10.1016/j.drudis.2024.103924. Epub 2024 Feb 22. Drug Discov Today. 2024. PMID: 38401878 Review.
References
-
- Hantusch B. Fundamentals of Vascular Biology. Springer; Berlin/Heidelberg, Germany: 2019. Morphological and functional characteristics of blood and lymphatic vessels; pp. 1–43.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

