Inhibition Effects and Mechanisms of Marine Compound Mycophenolic Acid Methyl Ester against Influenza A Virus
- PMID: 38786581
- PMCID: PMC11122424
- DOI: 10.3390/md22050190
Inhibition Effects and Mechanisms of Marine Compound Mycophenolic Acid Methyl Ester against Influenza A Virus
Abstract
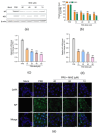
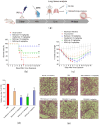
Influenza A virus (IAV) can cause infection and illness in a wide range of animals, including humans, poultry, and swine, and cause annual epidemics, resulting in thousands of deaths and millions of hospitalizations all over the world. Thus, there is an urgent need to develop novel anti-IAV drugs with high efficiency and low toxicity. In this study, the anti-IAV activity of a marine-derived compound mycophenolic acid methyl ester (MAE) was intensively investigated both in vitro and in vivo. The results showed that MAE inhibited the replication of different influenza A virus strains in vitro with low cytotoxicity. MAE can mainly block some steps of IAV infection post adsorption. MAE may also inhibit viral replication through activating the cellular Akt-mTOR-S6K pathway. Importantly, oral treatment of MAE can significantly ameliorate pneumonia symptoms and reduce pulmonary viral titers, as well as improving the survival rate of mice, and this was superior to the effect of oseltamivir. In summary, the marine compound MAE possesses anti-IAV effects both in vitro and in vivo, which merits further studies for its development into a novel anti-IAV drug in the future.
Keywords: Akt-mTOR-S6K pathway; MAE; anti-viral effect; influenza A virus; viral pneumonia.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Inhibition effects of novel polyketide compound PPQ-B against influenza A virus replication by interfering with the cellular EGFR pathway.Antiviral Res. 2017 Jul;143:74-84. doi: 10.1016/j.antiviral.2017.04.007. Epub 2017 Apr 13. Antiviral Res. 2017. PMID: 28414053
-
Inhibition of Influenza A Virus Infection by Fucoidan Targeting Viral Neuraminidase and Cellular EGFR Pathway.Sci Rep. 2017 Jan 17;7:40760. doi: 10.1038/srep40760. Sci Rep. 2017. PMID: 28094330 Free PMC article.
-
Inhibition of curcumin on influenza A virus infection and influenzal pneumonia via oxidative stress, TLR2/4, p38/JNK MAPK and NF-κB pathways.Int Immunopharmacol. 2018 Jan;54:177-187. doi: 10.1016/j.intimp.2017.11.009. Epub 2017 Nov 15. Int Immunopharmacol. 2018. PMID: 29153953
-
Plant extracts as a source of antiviral agents against influenza A virus.J Appl Microbiol. 2025 Mar 3;136(3):lxaf056. doi: 10.1093/jambio/lxaf056. J Appl Microbiol. 2025. PMID: 40058769 Review.
-
The Influenza A Virus Replication Cycle: A Comprehensive Review.Viruses. 2024 Feb 19;16(2):316. doi: 10.3390/v16020316. Viruses. 2024. PMID: 38400091 Free PMC article. Review.
Cited by
-
Marine natural product Methyl mycophenolate inhibits gastric cancer growth through regulating p53 and the downstream pathways.Cancer Cell Int. 2025 Jun 8;25(1):206. doi: 10.1186/s12935-025-03835-6. Cancer Cell Int. 2025. PMID: 40484944 Free PMC article.
-
Mycophenolate mofetil exerts broad-spectrum antiviral activity against coronaviruses including SARS-CoV-2.Virol J. 2025 Mar 4;22(1):56. doi: 10.1186/s12985-025-02673-2. Virol J. 2025. PMID: 40038695 Free PMC article.
References
-
- Pleschka S. Overview of influenza viruses. Curr. Top. Microbiol. Immunol. 2013;370:1–20. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

