Preparation and In Vitro Characterization of Lactococcus lactis-Loaded Alginate Particles as a Promising Delivery Tool for Periodontal Probiotic Therapy
- PMID: 38786639
- PMCID: PMC11121860
- DOI: 10.3390/jfb15050129
Preparation and In Vitro Characterization of Lactococcus lactis-Loaded Alginate Particles as a Promising Delivery Tool for Periodontal Probiotic Therapy
Abstract
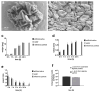
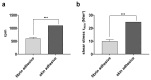
Probiotic microorganisms are used in a variety of food supplements and medical formulations to promote human health. In periodontal therapy, probiotics are mainly used in the form of gels, tablets or rinses that often tend to leak from the periodontal pocket, resulting in a strongly reduced therapeutic effect. In this pilot in vitro study, we present biodegradable alginate-based particles as an alternative, highly efficient system for a periodontal delivery of probiotic bacteria to the inflammation site. For this purpose, Lactococcus (L.) lactis was encapsulated using a standardized pump-controlled extrusion-dripping method. Time-dependent bacterial release in artificial saliva was investigated over 9 days. The effect of freeze drying was explored to ensure long-term storage of L. lactis-loaded particles. Additionally, the particles were bound to dentin surface using approved bioadhesives and subjected to shear stress in a hydrodynamic flow chamber that mimics the oral cavity in vitro. Thus, round particles within the range of 0.80-1.75 mm in radius could be produced, whereby the diameter of the dripping tip had the most significant impact on the size. Although both small and large particles demonstrated a similar release trend of L. lactis, the release rate was significantly higher in the former. Following lyophilization, particles could restore their original shape within 4 h in artificial saliva; thereby, the bacterial viability was not affected. The attachment strength to dentin intensified by an adhesive could resist forces between 10 and 25 N/m2. Full degradation of the particles was observed after 20 days in artificial saliva. Therefore, alginate particles display a valuable probiotic carrier for periodontal applications that have several crucial advantages over existing preparations: a highly stable form, prolonged continuous release of therapeutic bacteria, precise manufacturing according to required dimensions at the application site, strong attachment to the tooth with low risk of dislocation, high biocompatibility and biodegradability.
Keywords: alginate particles; periodontal health; probiotic therapy.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
An in vitro gastrointestinal model to evaluate the tolerance of encapsulated Lactobacillus and Lactococcus strains with synbiotic containing lactobionic acid via lyophilization technique to harsh gastric conditions during storage time.Eur J Pharm Biopharm. 2024 Apr;197:114147. doi: 10.1016/j.ejpb.2023.11.012. Epub 2023 Nov 14. Eur J Pharm Biopharm. 2024. PMID: 37967725
-
Microencapsulation of probiotics in hydrogel particles: enhancing Lactococcus lactis subsp. cremoris LM0230 viability using calcium alginate beads.Food Funct. 2016 Apr;7(4):1797-804. doi: 10.1039/c5fo00801h. Food Funct. 2016. PMID: 26611443
-
Solving the delivery of Lactococcus lactis: Improved survival and storage stability through the bioencapsulation with different carriers.J Food Sci. 2023 Apr;88(4):1495-1505. doi: 10.1111/1750-3841.16538. Epub 2023 Mar 20. J Food Sci. 2023. PMID: 36939001
-
Probiotics in tilapia (Oreochromis niloticus) culture: Potential probiotic Lactococcus lactis culture conditions.J Biosci Bioeng. 2022 Mar;133(3):187-194. doi: 10.1016/j.jbiosc.2021.11.004. Epub 2021 Dec 15. J Biosci Bioeng. 2022. PMID: 34920949 Review.
-
Encapsulated probiotic cells: Relevant techniques, natural sources as encapsulating materials and food applications - A narrative review.Food Res Int. 2020 Nov;137:109682. doi: 10.1016/j.foodres.2020.109682. Epub 2020 Sep 18. Food Res Int. 2020. PMID: 33233258 Review.
References
LinkOut - more resources
Full Text Sources

