Winter Hive Debris Analysis Is Significant for Assessing the Health Status of Honeybee Colonies (Apis mellifera)
- PMID: 38786906
- PMCID: PMC11121827
- DOI: 10.3390/insects15050350
Winter Hive Debris Analysis Is Significant for Assessing the Health Status of Honeybee Colonies (Apis mellifera)
Abstract
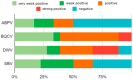
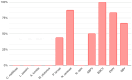
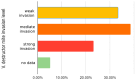
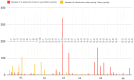
Honeybee diseases are one of the most significant and most common causes of honeybee colonies' weakness and death. An early diagnosis of subclinical infections is necessary to implement precautionary and control measures. Sampling debris from hive bottom boards is simple, non-invasive, and cheap. In this study, we collected winter debris samples in apiaries located in the continental part of Croatia. We used molecular methods, PCR and qPCR, for the first time to analyze those samples. Laboratory results were compared with the health condition and strength of honeybee colonies at an apiary in spring. Our study successfully identified the presence and quantity of various pathogens, including the presence of Vairimorpha spp. (Nosema spp.), quintefied Paenibacillus larvae, Acute Bee Paralysis Virus (ABPV), Black Queen Cell Virus (BQCV), Deformed Wing Virus (DWV), and Sacbrood Virus (SBV). However, our analysis did not detect Melissococcus plutonius, Crithidia mellificae, Lotmaria passim, and Aethina tumida. Samples of winter debris were also examined for the presence and quantification of the V. destructor mites, and their natural mite fall was observed in spring. Honeybee colonies were simultaneously infected by an average of four to six pathogens. Some observed honeybee colonies developed characteristic symptoms, while others did not survive the winter.
Keywords: Acute Bee Paralysis Virus; Aethina tumida; Black Queen Cell Virus; Crithidia mellificae; Deformed Wing Virus and Sacbrood Virus; Lotmaria passim; Melissococcus plutonius; PCR/qPCR; Paenibacillus larvae; Vairimorpha spp. (Nosema spp.); Varroa destructor; honeybee colony (Apis mellifera); samples of winter hive debris.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Radcliffe R.W., Seeley T.D. Looking to Nature to Solve the Health Crisis of Honey Bees. In: Kane T.R., Faux C.M., editors. Honey Bee Medicine for the Veterinary Practitioner. 1st ed. John Wiley and Sons; Hoboken, NJ, USA: 2021. pp. 1–20.
-
- Robinson W.E., Nowogrodzki R., Morse R.A. The value of honeybees as pollinators of US crops. Am. Bee J. 1989;129:477–487.
-
- Rollin O., Benelli G., Benvenuti S., Decourtye A., Wratten S.D., Canale A., Desneux N. Weed-insect pollinator networks as bioindicators of ecological sustainability in agriculture. A review. Agron. Sustain. Dev. 2016;36:8. doi: 10.1007/s13593-015-0342-x. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

