Exposure to Microcystin-LR Promotes Colorectal Cancer Progression by Altering Gut Microbiota and Associated Metabolites in APCmin/+ Mice
- PMID: 38787064
- PMCID: PMC11125743
- DOI: 10.3390/toxins16050212
Exposure to Microcystin-LR Promotes Colorectal Cancer Progression by Altering Gut Microbiota and Associated Metabolites in APCmin/+ Mice
Abstract
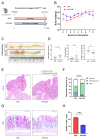
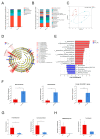
Microcystins (MCs), toxins generated by cyanobacteria, feature microcystin-LR (MC-LR) as one of the most prevalent and toxic variants in aquatic environments. MC-LR not only causes environmental problems but also presents a substantial risk to human health. This study aimed to investigate the impact of MC-LR on APCmin/+ mice, considered as an ideal animal model for intestinal tumors. We administered 40 µg/kg MC-LR to mice by gavage for 8 weeks, followed by histopathological examination, microbial diversity and metabolomics analysis. The mice exposed to MC-LR exhibited a significant promotion in colorectal cancer progression and impaired intestinal barrier function in the APCmin/+ mice compared with the control. Gut microbial dysbiosis was observed in the MC-LR-exposed mice, manifesting a notable alteration in the structure of the gut microbiota. This included the enrichment of Marvinbryantia, Gordonibacter and Family_XIII_AD3011_group and reductions in Faecalibaculum and Lachnoclostridium. Metabolomics analysis revealed increased bile acid (BA) metabolites in the intestinal contents of the mice exposed to MC-LR, particularly taurocholic acid (TCA), alpha-muricholic acid (α-MCA), 3-dehydrocholic acid (3-DHCA), 7-ketodeoxycholic acid (7-KDCA) and 12-ketodeoxycholic acid (12-KDCA). Moreover, we found that Marvinbryantia and Family_XIII_AD3011_group showed the strongest positive correlation with taurocholic acid (TCA) in the mice exposed to MC-LR. These findings provide new insights into the roles and mechanisms of MC-LR in susceptible populations, providing a basis for guiding values of MC-LR in drinking water.
Keywords: Apcmin/+ mice; bile acid; colorectal cancer; gut microbiota; microcystin-LR.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Exposure to microcystin-LR promotes the progression of colitis-associated colorectal cancer by inducing barrier disruption and gut microbiota dysbiosis.Ecotoxicol Environ Saf. 2024 Sep 1;282:116750. doi: 10.1016/j.ecoenv.2024.116750. Epub 2024 Jul 24. Ecotoxicol Environ Saf. 2024. PMID: 39053045
-
Systemic inflammatory response to daily exposure to microcystin-LR and the underlying gut microbial mechanisms.J Hazard Mater. 2024 Dec 5;480:135855. doi: 10.1016/j.jhazmat.2024.135855. Epub 2024 Sep 16. J Hazard Mater. 2024. PMID: 39303605
-
Microcystis toxin-mediated tumor promotion and toxicity lead to shifts in mouse gut microbiome.Ecotoxicol Environ Saf. 2020 Dec 15;206:111204. doi: 10.1016/j.ecoenv.2020.111204. Epub 2020 Aug 29. Ecotoxicol Environ Saf. 2020. PMID: 32871519
-
Microcystin-LR Induces Lipid Metabolism Disorder in Pelophylax nigromaculatus Tadpoles via the Gut-Liver Axis.Environ Sci Technol. 2025 May 20;59(19):9399-9411. doi: 10.1021/acs.est.4c12957. Epub 2025 May 8. Environ Sci Technol. 2025. PMID: 40337926
-
Research progress of microcystin-LR toxicity to the intestine, liver, and kidney and its mechanism.Environ Int. 2025 Jul;201:109547. doi: 10.1016/j.envint.2025.109547. Epub 2025 May 29. Environ Int. 2025. PMID: 40460709 Review.
Cited by
-
Galla chinensis alleviated liver damage induced by acetaminophen by regulating intestinal microbiota.Front Microbiol. 2025 Jul 1;16:1589946. doi: 10.3389/fmicb.2025.1589946. eCollection 2025. Front Microbiol. 2025. PMID: 40666811 Free PMC article.
-
Comparative Analysis of Gut Microbiota Responses to New SN-38 Derivatives, Irinotecan, and FOLFOX in Mice Bearing Colorectal Cancer Patient-Derived Xenografts.Cancers (Basel). 2025 Jul 7;17(13):2263. doi: 10.3390/cancers17132263. Cancers (Basel). 2025. PMID: 40647560 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

