Recovery of Ag(I) from Wastewater by Adsorption: Status and Challenges
- PMID: 38787130
- PMCID: PMC11125793
- DOI: 10.3390/toxics12050351
Recovery of Ag(I) from Wastewater by Adsorption: Status and Challenges
Abstract
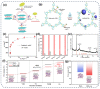
Untreated or inadequately treated silver-containing wastewater may pose adverse effects on hu-man health and the ecological environment. Currently, significant progress has been made in the treatment of Ag(I) in wastewater using adsorption methods, with adsorbents playing a pivotal role in this process. This paper provides a systematic review of various adsorbents for the recovery and treatment of Ag(I) in wastewater, including MOFs, COFs, transition metal sulfides, metal oxides, biomass materials, and other polymeric materials. The adsorption mechanisms of these materials for Ag(I) are elaborated upon, along with the challenges currently faced. Furthermore, insights into optimizing adsorbents and developing novel adsorbents are proposed in this study.
Keywords: Ag(I); adsorbent optimization; adsorbents; adsorption mechanism.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
Oil palm biomass as an adsorbent for heavy metals.Rev Environ Contam Toxicol. 2014;232:61-88. doi: 10.1007/978-3-319-06746-9_3. Rev Environ Contam Toxicol. 2014. PMID: 24984835 Review.
-
A novel green synthesis of MnO2-Coal composite for rapid removal of silver and lead from wastewater.Water Res. 2024 Jun 1;256:121526. doi: 10.1016/j.watres.2024.121526. Epub 2024 Apr 2. Water Res. 2024. PMID: 38583333
-
A critical review on adsorption and recovery of fluoride from wastewater by metal-based adsorbents.Environ Sci Pollut Res Int. 2022 Nov;29(55):82740-82761. doi: 10.1007/s11356-022-23416-8. Epub 2022 Oct 13. Environ Sci Pollut Res Int. 2022. PMID: 36224467 Review.
-
Recent advances and challenges on removal and recycling of phosphate from wastewater using biomass-derived adsorbents.Chemosphere. 2021 Sep;278:130377. doi: 10.1016/j.chemosphere.2021.130377. Epub 2021 Mar 28. Chemosphere. 2021. PMID: 33819886 Review.
-
Synthesis of novel magnetic pitch-based hypercrosslinked polymers as adsorbents for effective recovery of Ag+ with high selectivity.J Environ Manage. 2023 Aug 1;339:117763. doi: 10.1016/j.jenvman.2023.117763. Epub 2023 Apr 7. J Environ Manage. 2023. PMID: 37031597
References
-
- Birloaga I., Vegliò F. Overview on hydrometallurgical procedures for silver recovery from various wastes. J. Environ. Chem. Eng. 2018;6:2932–2938. doi: 10.1016/j.jece.2018.04.040. - DOI
-
- Yazdani-Ahmadabadi H., Felix D.F., Yu K., Yeh H.H., Luo H.D., Khoddami S., Takeuchi L.E., Alzahrani A., Abbina S., Mei Y., et al. Durable Surfaces from Film-Forming Silver Assemblies for Long-Term Zero Bacterial Adhesion without Toxicity. Acs Cent. Sci. 2022;8:546–561. doi: 10.1021/acscentsci.1c01556. - DOI - PMC - PubMed
-
- Pan X.H., Zu J.H., Diao J.J., Xue Y., Liu S.Y. Rapid and selective recovery of Ag(I) from simulative electroplating effluents by sulfydryl-rich covalent organic framework (COF-SH) with high adsorption capacity. Colloids Surf. A Physicochem. Eng. Asp. 2022;648:129156. doi: 10.1016/j.colsurfa.2022.129156. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

