Titanium Dioxide Nanoparticles Induce Maternal Preeclampsia-like Syndrome and Adverse Birth Outcomes via Disrupting Placental Function in SD Rats
- PMID: 38787146
- PMCID: PMC11125676
- DOI: 10.3390/toxics12050367
Titanium Dioxide Nanoparticles Induce Maternal Preeclampsia-like Syndrome and Adverse Birth Outcomes via Disrupting Placental Function in SD Rats
Abstract
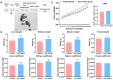
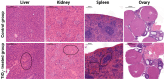
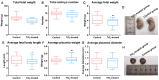
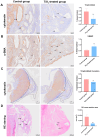
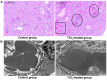
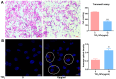
The escalating utilization of titanium dioxide nanoparticles (TiO2 NPs) in everyday products has sparked concerns regarding their potential hazards to pregnant females and their offspring. To address these concerns and shed light on their undetermined adverse effects and mechanisms, we established a pregnant rat model to investigate the impacts of TiO2 NPs on both maternal and offspring health and to explore the underlying mechanisms of those impacts. Pregnant rats were orally administered TiO2 NPs at a dose of 5 mg/kg body weight per day from GD5 to GD18 during pregnancy. Maternal body weight, organ weight, and birth outcomes were monitored and recorded. Maternal pathological changes were examined by HE staining and TEM observation. Maternal blood pressure was assessed using a non-invasive blood analyzer, and the urinary protein level was determined using spot urine samples. Our findings revealed that TiO2 NPs triggered various pathological alterations in maternal liver, kidney, and spleen, and induced maternal preeclampsia-like syndrome, as well as leading to growth restriction in the offspring. Further examination unveiled that TiO2 NPs hindered trophoblastic cell invasion into the endometrium via the promotion of autophagy. Consistent hypertension and proteinuria resulted from the destroyed the kidney GBM. In total, an exposure to TiO2 NPs during pregnancy might increase the risk of human preeclampsia through increased maternal arterial pressure and urinary albumin levels, as well as causing fetal growth restriction in the offspring.
Keywords: autophagy; placenta development; preeclampsia-like syndrome; pregnant model; titanium dioxide nanoparticles; trophoblastic cell function.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Maternal exposure to nano-titanium dioxide impedes fetal development via endothelial-to-mesenchymal transition in the placental labyrinth in mice.Part Fibre Toxicol. 2023 Dec 11;20(1):48. doi: 10.1186/s12989-023-00549-3. Part Fibre Toxicol. 2023. PMID: 38072983 Free PMC article.
-
Maternal exposure to titanium dioxide nanoparticles during pregnancy and lactation alters offspring hippocampal mRNA BAX and Bcl-2 levels, induces apoptosis and decreases neurogenesis.Exp Toxicol Pathol. 2017 Jul 5;69(6):329-337. doi: 10.1016/j.etp.2017.02.006. Epub 2017 Feb 27. Exp Toxicol Pathol. 2017. PMID: 28254502
-
Maternal, placental, and fetal distribution of titanium after repeated titanium dioxide nanoparticle inhalation through pregnancy.Placenta. 2022 Apr;121:99-108. doi: 10.1016/j.placenta.2022.03.008. Epub 2022 Mar 12. Placenta. 2022. PMID: 35305398 Free PMC article.
-
A key moment for TiO2: Prenatal exposure to TiO2 nanoparticles may inhibit the development of offspring.Ecotoxicol Environ Saf. 2020 Oct 1;202:110911. doi: 10.1016/j.ecoenv.2020.110911. Epub 2020 Jul 8. Ecotoxicol Environ Saf. 2020. PMID: 32800246 Review.
-
Titanium dioxide nanoparticles: some aspects of toxicity/focus on the development.Endocr Regul. 2015 Apr;49(2):97-112. doi: 10.4149/endo_2015_02_97. Endocr Regul. 2015. PMID: 25960011 Review.
References
-
- Gulson B., McCall M.J., Bowman D.M., Pinheiro T. A review of critical factors for assessing the dermal absorption of metal oxide nanoparticles from sunscreens applied to humans, and a research strategy to address current deficiencies. Arch. Toxicol. 2015;89:1909–1930. doi: 10.1007/s00204-015-1564-z. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

