The Establishment of a Novel γ-Interferon In Vitro Release Assay for the Differentiation of Mycobacterial Bovis-Infected and BCG-Vaccinated Cattle
- PMID: 38787170
- PMCID: PMC11125845
- DOI: 10.3390/vetsci11050198
The Establishment of a Novel γ-Interferon In Vitro Release Assay for the Differentiation of Mycobacterial Bovis-Infected and BCG-Vaccinated Cattle
Abstract
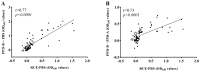
BCG vaccination is increasingly reconsidered in the effective prevention of bovine tuberculosis (bTB). However, the primary challenge in BCG vaccination for cattle is the lack of a technique for differentiating between infected and vaccinated animals (DIVA). This study aimed to establish a novel DIVA diagnostic test based on an interferon-gamma in vitro release assay (IGRA). The plasmid encoding three differential antigens (Rv3872, CFP-10, and ESAT-6) absent in BCG genes but present in virulent M. bovis was previously constructed. Thus, a recombinant protein called RCE (Rv3872, CFP-10, and ESAT-6) was expressed, and an RCE-based DIVA IGRA (RCE-IGRA) was established. The RCE concentration was optimized at 4 μg/mL by evaluating 97 cattle (74 of which were bTB-positive, and 23 were negative) using a commercial IGRA bTB diagnostic kit. Further, 84 cattle were tested in parallel with the RCE-IGRA and commercial PPD-based IGRA (PPD-IGRA), and the results showed a high correlation with a kappa value of 0.83. The study included BCG-vaccinated calves (n = 6), bTB-positive cattle (n = 6), and bTB-negative non-vaccinated calves (n = 6). After 3 months post-vaccination, PPD-IGRA generated positive results in both vaccinated and infected calves. However, RCE-IGRA developed positive results in infected calves but negative results in vaccinated calves. In conclusion, this DIVA method has broad prospects in differentiating BCG vaccination from natural infection to prevent bTB.
Keywords: BCG; bovine tuberculosis (bTB); differential antigens; differentiation of the infected from vaccinated animals (DIVA); interferon-gamma (IFN-γ) in vitro release assay (IGRA); regions of difference (RDs); vaccination.
Conflict of interest statement
The authors declare no potential conflict of interest in the research, authorship, and/or publication of this article.
Figures



Similar articles
-
Comparative performance of tuberculin and defined-antigen cocktails for detecting bovine tuberculosis in BCG-vaccinated cattle in natural settings.Sci Rep. 2025 Feb 7;15(1):4564. doi: 10.1038/s41598-025-85389-1. Sci Rep. 2025. PMID: 39915566 Free PMC article.
-
Field evaluation of specific mycobacterial protein-based skin test for the differentiation of Mycobacterium bovis-infected and Bacillus Calmette Guerin-vaccinated crossbred cattle in Ethiopia.Transbound Emerg Dis. 2022 Jul;69(4):e1-e9. doi: 10.1111/tbed.14252. Epub 2021 Aug 19. Transbound Emerg Dis. 2022. PMID: 34331511 Free PMC article.
-
A Defined Antigen Skin Test That Enables Implementation of BCG Vaccination for Control of Bovine Tuberculosis: Proof of Concept.Front Vet Sci. 2020 Jul 24;7:391. doi: 10.3389/fvets.2020.00391. eCollection 2020. Front Vet Sci. 2020. PMID: 32793643 Free PMC article.
-
[Evolution of IGRA researches].Kekkaku. 2008 Sep;83(9):641-52. Kekkaku. 2008. PMID: 18979999 Review. Japanese.
-
DIVA reagents for bovine tuberculosis vaccines in cattle.Expert Rev Vaccines. 2011 Jul;10(7):1083-91. doi: 10.1586/erv.11.22. Expert Rev Vaccines. 2011. PMID: 21806401 Review.
References
-
- Srinivasan S., Conlan A.J.K., Easterling L.A., Herrera C., Dandapat P., Veerasami M., Ameni G., Jindal N., Raj G.D., Wood J., et al. A Meta-Analysis of the Effect of Bacillus Calmette-Guérin Vaccination Against Bovine Tuberculosis: Is Perfect the Enemy of Good? Front. Vet. Sci. 2021;8:637580. doi: 10.3389/fvets.2021.637580. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

