Gastric Cancer, Immunotherapy, and Nutrition: The Role of Microbiota
- PMID: 38787209
- PMCID: PMC11124250
- DOI: 10.3390/pathogens13050357
Gastric Cancer, Immunotherapy, and Nutrition: The Role of Microbiota
Abstract
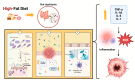
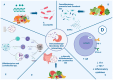
Immune checkpoint inhibitors (ICI) have revolutionized the treatment of gastric cancer (GC), which still represents the third leading cause of cancer-related death in Western countries. However, ICI treatment outcomes vary between individuals and need to be optimized. Recent studies have shown that gut microbiota could represent a key influencer of immunotherapy responses. At the same time, the nutritional status and diet of GC patients are also predictive of immunotherapy treatment response and survival outcomes. The objective of this narrative review is to gather recent findings about the complex relationships between the oral, gastric, and gut bacterial communities, dietary factors/nutritional parameters, and immunotherapy responses. Perigastric/gut microbiota compositions/functions and their metabolites could be predictive of response to immunotherapy in GC patients and even overall survival. At the same time, the strong influence of diet on the composition of the microbiota could have consequences on immunotherapy responses through the impact of muscle mass in GC patients during immunotherapy. Future studies are needed to define more precisely the dietary factors, such as adequate daily intake of prebiotics, that could counteract the dysbiosis of the GC microbiota and the impaired nutritional status, improving the clinical outcomes of GC patients during immunotherapy.
Keywords: H. pylori; ICI; diet; gastric cancer; gut microbiota; immunotherapy; immunotherapy response; malnutrition; muscle mass; nutrition; nutritional status.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Ekmekciu I., von Klitzing E., Fiebiger U., Escher U., Neumann C., Bacher P., Scheffold A., Kühl A.A., Bereswill S., Heimesaat M.M. Immune responses to broad-spectrum antibiotic treatment and fecal microbiota transplantation in mice. Front. Immunol. 2017;8:397. doi: 10.3389/fimmu.2017.00397. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

